CHAPTER 35 Clinical Reproductive Physiology of the Cow
Reproduction drives the production cycle in both beef and dairy enterprises and is of major economic consequence. Maximizing the reproductive potential in cattle requires the understanding and application of many principles basic to the various disciplines of animal and veterinary science, including genetics, nutrition, physiology, and theriogenology, as well as management intervention.
ONSET OF SEXUAL MATURATION
Increasing reproductive efficiency in beef and dairy herds depends on the timely introduction of replacement heifers into the breeding herd. Heifers should have their first calf by 2 years of age. Maximizing the proportion of beef heifers that calve early in the calving season leads to heavier calf weaning weights, allows for timely rebreeding, and increases their herd longevity. To maximize lifetime milk production in a dairy operation, replacements also must calve as 2-year-olds in order to recoup rearing costs. Achieving this goal in either type of production unit requires appropriate heifer development and timely onset of puberty and first conception.
Factors Influencing Onset of Puberty
The onset of puberty, as well as of nonpubertal estrus, is influenced by several factors, including age, genotype, season, body weight, nutrition, and social rearing environment.1
Season
Although cattle are not considered to be seasonal breeders, evidence indicates that season of birth may alter timing of puberty. When heifers were fed similarly in controlled environmental chambers, those born in the autumn reached puberty earlier than those born in the spring.2 Heifers born late within a spring calving season, however, were younger and lighter at puberty, probably because of improved forage quality and its effects on milk availability from their dams and digestibility of forages obtained by their grazing.
Body Weight and Nutrition
Underfeeding or overfeeding heifers has significant consequences on their development. Underfeeding of growing heifers may result in delayed puberty, subnormal conception rates, underdeveloped mammary glands, and greater incidence of calving problems.3 Overfeeding often results in weak expression of estrus, subnormal conception rates, high embryonic mortality, decreased mammary gland development, and reduced milk production.3 Dairy heifers fed 115% of all 1989 National Research Council nutrient requirements for heifers to gain 0.7 kg/day from 3 to 24 months of age were younger at puberty, were heavier at calving, had larger heart girth, were longer, were not excessively fat at calving, and calved at younger ages. Nutrient requirements suggested by the National Research Council should serve as minimum guidelines.
Social Rearing Environment
In some feral mammalian species, the physical presence of the male decreases age at puberty in contemporary females. Presence of mature bulls and adult cycling cows has altered age at puberty of heifers in some but not all studies. Increased proportions of prepubertal beef heifers treated oronasally with bull urine reached puberty earlier than water-treated controls.3
Endocrine Mechanisms
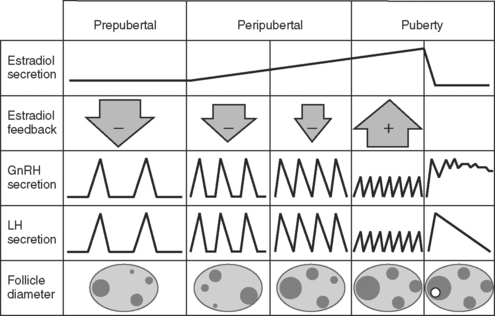
The events necessary to initiate the first pubertal estrus occur by an organized and interdependent cascade of changes in the central nervous system, hypothalamus, pituitary, and ovaries during maturation.4 These events are summarized in Figure 35-1. As early as 4 months of age, the pituitary is capable of releasing luteinizing hormone (LH) in response to injections of gonadotropin-releasing hormone (GnRH). Likewise, the ovaries respond to exogenous and endogenous (GnRH-induced) gonadotropins by increasing the production of ovarian steroids.4
Early ovarian follicular development is evident. In fact, intrarectal ultrasonography shows that groups of follicles develop in regular repeating patterns (waves), beginning as early as 2 weeks of age, similar to what is observed in adult females during the estrous cycle and pregnancy4 (see discussion of follicular dynamics below). The largest follicle, or dominant follicle, within each follicular wave of developing follicles progressively increases in maximal diameter from 2 to 34 weeks of age, parallel to increasing numbers of follicles detected in each follicular wave.4 The combined evidence of early established pituitary and ovarian competence suggests that hypothalamic inactivity (lack of GnRH pulses secreted into the hypophyseal portal vessels) is responsible for maintaining the prepubertal status. The mechanism by which the hypothalamus remains relatively inactive has not been elucidated in cattle. Maturation of the GnRH pulse system (pulse generator) in the hypothalamus may necessitate other central nervous system involvement. Increased hypothalamic turnover and decreased sensitivity to various neurotransmitters occur in rodents as puberty is imminent. Specifically, increased turnover of norepinephrine and dopamine normally accompanies pulses of LH in the rat. Endogenous opioids may be involved in inhibiting the requisite LH pulse frequency, because administering an opioid receptor antagonist (naloxone) to 4-week-old heifers induced LH pulses, with the response decreasing as heifers approached 32 weeks of age.4
Evidence in ewes and heifers suggests that the prepubertal GnRH pulse generator is highly sensitive to the negative feedback of ovarian estrogens, such that estradiol inhibits secretory pulses of LH and probably those of GnRH as well (see Fig. 35-1). This phenomenon was described earlier in rats and is known as the gonadostat theory of puberty.4 This theory suggests that the threshold to the negative feedback of estrogen increases as puberty approaches, accounting for a decreased sensitivity of the hypothalamus to the negative feedback of estrogen. Thus, the inhibitory mechanism modulating pulsatile release of GnRH and LH decreases, resulting in an increasing frequency of pulses, which normally are observed as puberty approaches (see Fig. 35-1). The pulse frequency of LH gradually increases between day 126 and day 14 before puberty, whereas concentrations of estradiol receptors within the hypothalamus decline.
Endogenous LH pulses occur at a frequency of one to four per 24 hours during the prepubertal period. As during the estrous cycle, the frequency of LH pulses sufficient to “drive” follicular maturation to ovulation must increase to approximately one pulse per hour. For 3 weeks before puberty, blood concentrations of progesterone seldom rise above 0.5 ng/ml (indicative of significant luteal tissue in the ovaries and little or no negative feedback of progesterone on LH secretion). Therefore, the frequency of LH pulses seems to be insufficient to support follicular maturation and ovulation during this period.4
The transition into puberty occurs for 2 to 4 weeks before first ovulation. This transient stage is characterized by an increased frequency of LH pulses and ovulation, as determined by a significant increase in blood concentrations of progesterone. In most cases, heifers in this transitional period exhibit one or two short-lived (duration of 2 to 5 days) elevations in blood progesterone of lower magnitude than what is observed in the normal estrous cycle. These transient increases in progesterone are preceded by LH pulses, the source of which may be luteinized ovarian follicles, and may be involved in preparing the uterus for the possibility of pregnancy and/or the establishment of normal patterns of GnRH and LH pulses characteristic of cycling females. The first major preovulatory LH surge occurs only after earlier prepubertal rises in progesterone, with a behavioral estrus observed in some heifers at this time. Puberty has been induced by administering a progestin as an ear implant containing norgestomet for 9 days,3 in addition to an intramuscular injection containing both norgestomet and estradiol valerate, or as a progesterone-releasing intravaginal insert. Similar success has been reported when melengestrol acetate was fed for 7 to 16 days.3 The effectiveness of these treatments depends on attainment of adequate body weight in heifers, characteristic of that necessary at normal puberty for their genotype.
PHYSIOLOGY OF THE ESTROUS CYCLE
General Traits
Once puberty occurs, estrous cycles generally continue unabated unless pregnancy is established or nutritional conditions are limited severely. Estrous cycles typically are 3 weeks in duration but normally may range from 17 to 25 days. The period of estrus may range from 2 to 50 hours in duration but averages 12 to 18 hours under most conditions. Ovulation occurs approximately 24 to 30 hours after the onset of estrus, with the first signs of estrus usually coinciding with the beginning of the preovulatory surge of LH and follicle-stimulating hormone (FSH). Durations of estrous cycles are often 1 to 2 days shorter in heifers than in cows. High seasonal temperatures do not seem to alter duration of cycles but may reduce the duration of estrus and decrease blood flow to the reproductive tract and may alter concentrations of various reproductive hormones.2
Some important differences in these basic traits of estrus have been observed between Bos taurus and Bos indicus breeds. Specifically, Bos indicus females are reported to have periods of estrus of shorter duration, shorter intervals from the onset of estrus to ovulation, reduced magnitude of the preovulatory concentration of the LH surge, smaller corpora lutea, and lower luteal-phase concentrations of progesterone.1,3
Endocrine Patterns
For purposes of describing the interactions of hormones secreted by the hypothalamus, pituitary, ovaries, and uterus that are involved in the orchestration of sexual behavior and normal endocrinology of the estrous cycle, the cycle can be divided into three endocrine periods: (1) pregonadotropin surge, (2) postgonadotropin surge, and (3) luteal phase.5
Pregonadotropin Surge
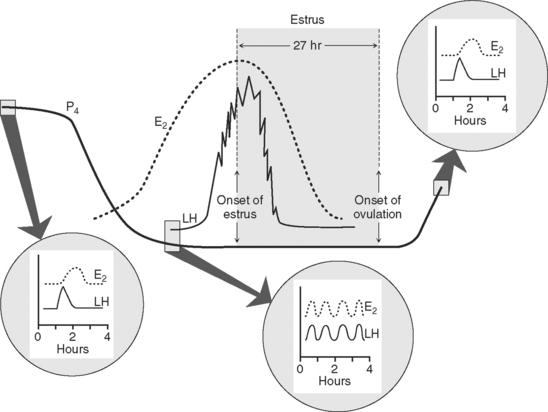
The period of the pregonadotropin surge begins with the demise of the corpus luteum and ends with the preovulatory surge of LH and FSH, which initiate ovulation of a mature follicle 24 to 30 hours later (Fig. 35-2). Concentrations of progesterone rapidly decline as those of estradiol begin to increase concurrently with the accelerated growth of the preovulatory follicle. As progesterone declines, the baseline concentration of LH increases,5 the frequency of LH pulses increases from about one pulse every 4 to 6 hours to one pulse every hour, but the amplitude of LH pulses declines5 (all because of reduced negative feedback by progesterone on the hypothalamus and pituitary). This change in LH secretion, which coincides with a parallel pulsing of estradiol secreted into the venous effluent of the ovary bearing the preovulatory follicle, can be monitored in jugular blood by frequently collected blood samples (see Fig. 35-2). Although follicular growth may be stimulated by FSH alone, a combination of LH and FSH has been shown to induce maximal synthesis of estradiol in vitro.6 Thus, both gonadotropins influence follicular development and subsequent secretion of estradiol. Estradiol appears to exert inhibitory effects on FSH release, because when it is administered to ovariectomized heifers, FSH is reduced to precastration concentrations. Furthermore, when titers of estradiol are at their peak just before the preovulatory surge, concentrations of FSH are lowest.5
A carefully orchestrated cascade of increasing LH pulses and elevated titers of estradiol eventually culminates in the onset of estrus, with estradiol triggering the initiation of both estrous behavior and the preovulatory surge of LH (see Fig. 35-2). Estradiol apparently triggers more frequent pulsatile GnRH secretion by the hypothalamus and enhanced pituitary responsiveness to each priming GnRH pulse. Blocking the rise in estradiol by chemical or immunologic means can block the occurrence of the gonadotropin surge.5 Likewise, concentrations of progesterone must be low before estradiol can induce the gonadotropin surge, because administering progesterone during the period before the surge can block it. The elevation of LH and FSH (surge) usually endures for 8 to 10 hours. Termination of the surge is due to refractoriness of the pituitary to GnRH (decreased concentration [down-regulation] of pituitary GnRH receptors) and depletion of the releasable pool of pituitary gonadotropin. This hypothesis is substantiated by two observations: (1) When heifers are ovariectomized shortly after the onset of estrus and the preovulatory surge, a delay in the classic postcastration rise in LH is observed, and (2) injections of GnRH within 12 to 16 hours of the onset of the surge result in the release of only marginal amounts of gonadotropin.
Postgonadotropin Surge
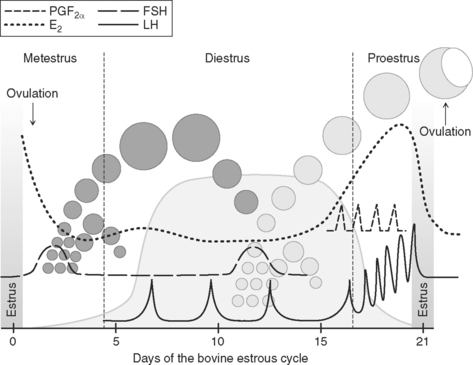
The postgonadotropin surge period is characterized by declining titers of estradiol in blood, after their peak at the onset of estrus, as follicular luteinization ensues (Fig. 35-3). The process of ovulation has been likened to mechanisms associated with an inflammatory reaction.7 The process of follicular rupture is regulated by a host of intrafollicular mediators. For example, ovulation can be blocked by intrafollicular administration of various prostaglandin enzyme inhibitors or antiserum to various prostaglandins or their regulatory enzymes.5 Inter–theca cell capillaries and the basement membrane of the follicle are disturbed at ovulation, accounting for some hemorrhage, and thus allowing both capillaries and theca cells to pervade the ruptured follicle. Follicular content of estradiol declines rapidly as progesterone content begins to increase, because both theca and granulosa cells differentiate into luteal cells (luteinize) that form the corpus luteum. Theca cells appear to develop into smaller-diameter cells, known as small luteal cells, and the granulosa cells become large luteal cells as the corpus luteum develops.8
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree