CHAPTER 87 Clinical Reproductive Physiology of Ewes
REPRODUCTIVE PATTERNS
The ewe is seasonally polyestrous, with breeds derived from northern latitudes having a defined breeding season spanning the fall and winter months. Breeds developed closer to the Equator show less seasonality. The estrous cycle of the ewe is usually 16 to 17 days long, with estrus lasting approximately 30 to 36 hours. Spontaneous ovulation occurs late in estrus. Gestation length is approximately 147 days, with minor variations between breeds.1
REPRODUCTIVE CYCLE
Folliculogenesis
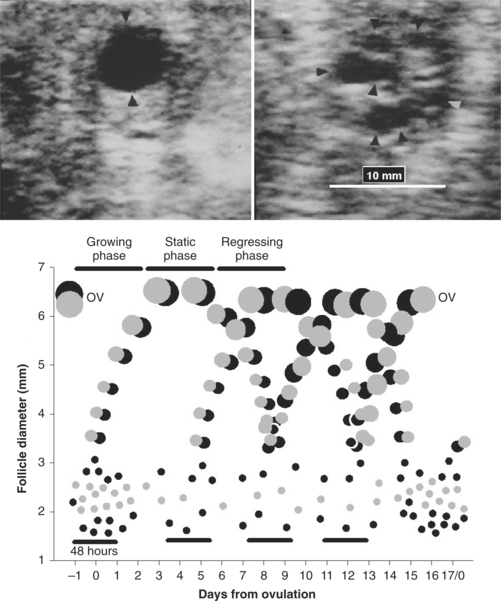
A resting pool of primordial follicles (containing primary oocytes) is established in the ewe prenatally, and in the adult ewe 3 to 4 follicles move from this pool and are committed to a growing pool (primary follicles) every day.2 Although luteinizing hormone (LH) receptors are present in the theca and follicle-stimulating hormone (FSH) receptors are in granulosa cells quite early in follicular development (tertiary or preantral follicles of about 0.1 mm in diameter), folliculogenesis appears to be gonadotropin independent in sheep up to the 1- to 2-mm stage (early antral follicles). Most follicles are lost to atresia in a process of programmed cell death (apoptosis), particularly at the preantral and antral stages of development. Recent use of transrectal ultrasonography has allowed description of antral follicle dynamics (follicles with diameter > 2 mm) in the ewe (Fig. 87-1).3 During the estrous cycle, antral follicles emerge or grow from the pool of follicles of 1 to 2 mm in diameter, approximately every 4 days (3–4 follicle waves per estrous cycle). The ovulatory follicle emerges around day 12 of the cycle. Unlike in cattle, the wave-like production of larger antral follicles in the ewe is not reflected in any change in the total numbers of small antral follicles (1–3 mm) in the ovary, apart from a small increase around ovulation.3–5 In each wave, follicles reach a maximum diameter of 4 to 7 mm; maximum diameter is lower in prolific compared to nonprolific ewes. The largest, nonovulatory follicles of waves have a life-span of 5 to 12 days (emergence to regression or atresia), and life-span is longer for follicles emerging early in the cycle, before luteal progesterone secretion is fully established. Large antral follicle life-span in the ewe consists of a 2- to 4-day growing phase, a static phase (no apparent change in size, 1–4 days), and regressing phase (1–5 days). Each follicular wave emerges after the antral follicles of the previous wave have ceased active growth or are regressing in the ewe. However, in the ewe, there is little evidence for follicular dominance as seen in cattle, except perhaps in the ovulatory wave, where growth of the ovulatory follicle in the ewe exceeds that of other follicles in the wave.4,6 Based on earlier, non-ultrasonographic studies, it was suggested that prolific ewes ovulated more follicles by reducing follicle atresia, recruiting more antral follicles into final growth and development, or by having a wider window of time over which ovulatory follicles were allowed to emerge.2 In comparing nonprolific ewes to the prolific Finn, using ultrasonography, it was clear that the increased ovulation rate was due to the ovulation of more follicles from follicle waves emerging before the final wave of the cycle (extended period of recruitment).3 Ovulation rate also increases from early to midbreeding season.1
In the cyclic ewe, the day of emergence of each follicle wave is synchronized to a small peak in serum concentrations of FSH (see Fig. 87-1).3 Serum concentrations of estradiol increase from follicle wave emergence and peak when the largest follicle of a wave reaches its maximum size. Antral follicles of prolific breeds of sheep have fewer granulosa cells than those of nonprolific breeds, but steroidogenic potential is greater as serum concentrations of estradiol can be greater in prolific compared to nonprolific ewes throughout the cycle.2 Termination of follicle growth and decreased secretion of estradiol could provide a signal for the subsequent peak in serum FSH concentrations that precedes and may induce the next wave of follicle emergence.3,7 Although inhibin specifically regulates FSH secretion, it is probably less involved in regulating peaks in FSH secretion as inhibin is secreted from a wide size range of follicles. The duration and amplitude of peaks of FSH and estradiol do not vary across the estrous cycle.3 LH pulse frequency is higher early in the cycle, before serum concentrations of progesterone have reached a maximum,8 and this could explain the longer life-span of the first follicle wave of the cycle.3 Interestingly, although mean serum FSH is greatest at wave emergence, FSH pulse frequency increases during the follicle growth phase of follicle waves.7 In the ewe, growth of follicles beyond 2 mm in diameter is FSH dependent, but the larger follicles of waves can transfer their dependence from FSH to LH (LH receptors develop in granulosa cells).2 This probably explains the ability of the preovulatory follicle to grow when serum FSH concentrations are at their lowest point in the cycle. The recent application of transrectal ovarian ultrasonography to the study of antral follicle dynamics in the ewe, described previously, promises to produce simple methods to increase ovulation rate and to synchronize follicle waves and ovulation to facilitate high fertility fixed-time insemination.3
In addition to endocrine regulation, follicular growth is subject to intraovarian control mechanisms. Insulin-like growth factors (IGFs) stimulate follicle growth and enhance gonadotropin responsiveness. The mRNA encoding IGF-II has been found in the theca of ovine follicles, but it is unclear if IGF-I is actually produced in the follicle or is supplied by production from the liver. Interestingly, a family of IGF binding proteins (IGFBPs) regulates the biologic activity of the IGFs, and a decrease in concentration of binding protein in antral follicles, as they grow, may enhance the biologic activity IGFs and gonadotropins. Inhibins and activins, members of the TGFβ superfamily, are produced by the growing follicle.9 Inhibin has an α subunit coupled to either a βA or βB subunit; activins are dimers of βB, βA, or βB/βA. Activins promote granulosa cell proliferation and differentiation and estrogen production and are anti-atretogenic. Follistatin, an activin binding protein, can oppose the effects of activin. Inhibin α and inhibin/activin βA subunits are expressed in increasing amounts as antral follicles mature in the ewe; however, the local roles of inhibin are not as clear. Other potential regulators of follicle development are transforming growth factors α and β, epidermal growth factor (EGF) and fibroblast growth factor (FGF).
The antral follicle(s) emerging near the end of the ovine estrous cycle and destined to ovulate does not necessarily grow any larger than nonovulatory follicles of earlier waves, nor does it produce more estrogen.3 The ovulatory follicle undergoes final development largely dependent on LH, and the preovulatory LH surge causes ovulation, luteinization, and maturation of the primary oocyte to a secondary oocyte.2 Recent work in the ewe has shown that the preovulatory LH signal causes secretion of plasminogen activator from the ovarian and ovulatory follicle wall.10 Plasmin activates collegenases, which disrupt the ovarian and follicle wall; plasmin also causes release of tumor necrosis factor alpha (TNFα), an apoptotic agent.
Luteal Function
The collapsed, ovulated follicle contains a blood clot and little luteal tissue. This structure grows rapidly and organizes; it is called a corpus hemorrhagicum until day 4 or 5 of the cycle, when it starts to produce significant amounts of progesterone, and thereafter it is called a corpus luteum. In Western white-faced ewes, serum progesterone concentrations increase to around day 12 of the cycle and then decline to day 16; however, from days 8 to 13 serum progesterone concentrations do not differ significantly (see Fig. 87-1). Based on recent ultrasonographic data from Western white-faced ewes,11 luteal volume increased to day 10 after estrus and then declined to day 17 of the cycle; however, luteal volumes did not differ significantly between days 8 and 13. Therefore, in Western white-faced ewes, a rapid increase in serum concentrations of progesterone preceded a marked increase in luteal volume. At luteolysis, functional and structural luteal regression seemed to commence coincidentally but the decline in serum concentrations of progesterone was somewhat sharper than for luteal volume. Prolific Finn ewes produced more but smaller corpora lutea and had lower serum concentrations of progesterone compared to Western white-faced ewes.11 Interestingly, ultrasonographic studies in the ewe have revealed luteinized follicles and short-lived corpora lutea developing in parallel with normal corpora lutea.3,7,11 In two ewes with the estrus cycle of 23 days each, normal life-span corpora lutea appeared to almost completely regress and then redevelop for a further luteal phase. In addition, serum progesterone concentrations vary with season; concentrations are greater in midbreeding season compared to the early or late season.11
Luteolysis is caused by pulses of prostaglandin F2α (PGF2α) from the endometrium of the uterine horn ipsilateral to the corpus luteum, between days 14 and 17 of the estrous cycle.12 The PGF2α is transported by a countercurrent exchange between the uterine vein and ovarian artery and the lymphatics are also involved. The pulsed release of PGF2α is driven by pulsed secretion of oxytocin from the corpus luteum and perhaps the posterior pituitary. What actually initiates the pulsed secretion of oxytocin and PGF2α is unclear. For oxytocin to stimulate PGF2α secretion, the development of endometrial oxytocin receptors is pivotal. Development of oxytocin receptors is blocked until days 10 to 12 of the cycle by progesterone. However, progesterone eventually down-regulates its own receptors, allowing an increase in estradiol receptors; estradiol is then able to stimulate production of oxytocin receptors.12 Prostaglandin F2α can cause functional luteolysis (loss of progesterone secretory ability) through receptors on large luteal cells and by disrupting cholesterol transport across mitochondrial membranes.12 PGF2α can also cause the release of endothelin 1 from endothelial cells in the corpus luteum. Endothelin 1 reduces progesterone secretion from luteal cells as well as causing vasoconstriction. Endothelin 1 may also attract monocytes to the corpus luteum and the release of transforming growth factor α from macrophages could be an early signal for apoptosis and structural regression of the corpus luteum. Prostaglandins (10–15 mg PGF2α IM) have been used to cause luteolysis and synchronize estrus in ewes; the corpus luteum is only responsive from day 4 of the cycle.1 Estrus occurs about 40 hours after PGF2α but the estrous response and fertility to breeding with PGF2α treatment are more variable than with other methods of estrous synchronization.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree