CHAPTER 98 Clinical Reproductive Physiology and Endocrinology of Sows: Mating Management
GENETIC SYSTEM
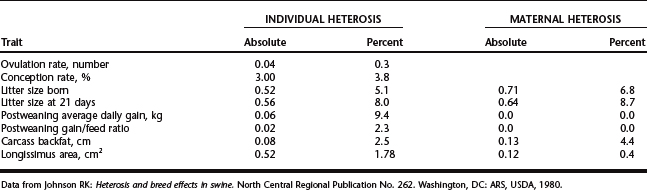
Heterosis tends to be highest for traits with the lowest heritability, those most susceptible to environmental variation. Reproductive and fitness performance, therefore, benefits most from heterosis. This includes embryo survival, litter size born, neonatal survival, and so on. For traits such as neonatal survival, a crossbred pig benefits from individual heterosis, and if the dam is crossbred and produces more milk, the piglet also benefits from maternal heterosis. A summary of heterosis values for some economically important traits is presented in Table 98-1.
Heterosis may be thought of as recovery from mild inbreeding associated with development of breeds or lines. Accordingly, it is not surprising that heterosis is beneficial to the pig for all traits, and to the producer for most traits, backfat being the exception. In rare instances, heterosis may not justify crossbreeding. If only two lines of pigs were available for use in a maternal line, one with a litter size of 8 and the other with 10 pigs, an F1 hybrid, with heterosis, would be expected to have (8 + 10) ÷ 2 + 10% heterosis = 9.9 pigs. Although this litter size is better than the parental mean, it is inferior to that of the better parent.
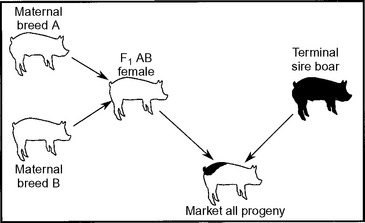
Figure 98-1 shows a crossbreeding system that maximizes heterosis and breed complementation. For this system to work, the F1 AB female will need to be either produced on farm or purchased. Systems purchasing the F1 AB maternal line female are referred to as parent systems. Those purchasing the pure breed A and breed B to produce F1 AB females are referred to as grandparent systems. Great-grandparent systems are similar but use an F1 female instead of the A or B breed. On-farm production of replacement females typically requires a commitment of 10% to 20% of the sows for replacement gilt production. Semen can be purchased and artificial insemination (AI) used to eliminate the need to maintain all of the boar lines.
REPRODUCTIVE ANATOMY
External Genitalia
Male pseudohermaphroditism can occur in pigs. Animals with this condition usually have female external genitalia but also have testes, or they may have gonadal tissue of both sexes. The testes may be located subcutaneously within the scrotal area but often are located within the abdomen. Male pseudohermaphrodites often have a “fishhook” or “sky-tipped” vulva.
ESTROUS CYCLES
Endocrinology
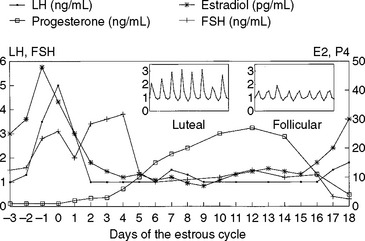
Changes in reproductive hormones during the estrous cycle have been well characterized2–4 (Fig. 98-2). The length of the estrous cycle in swine is approximately 21 days. For the purposes of this discussion, the cycle is divided into the follicular phase (proestrus), the ovulatory phase (estrus), and the luteal phase (diestrus). Patterns of circulating hormones and intraovarian events are described.
The follicular phase is the period from luteal regression to ovulation. Luteal regression, as indicated by a decline in circulating progesterone concentrations, occurs between 13 and 15 days after the previous estrus. This decline in progesterone is caused by the uterine luteolysin, prostaglandin F2α (PGF2α), in the utero-ovarian circulation. During this time, follicles destined to ovulate enter a period of accelerated growth resulting from increased frequency of episodic luteinizing hormone (LH) release after the progesterone decline. Increases in LH receptors on ovarian graafian follicles promote increased synthesis and secretion of follicular estradiol. Estradiol concentrations peak in serum between days 18 and 20 of the estrous cycle, due to increased follicular aromatase enzyme activity. Approximately 40% of follicles present on days 15 to 16 of the cycle undergo atresia or degeneration before the LH surge (day 20 or 21) (Fig. 98-3). Follicles that go on to ovulate reach diameters of 5 to 12 mm before ovulation.
The rise in estradiol during the follicular phase is responsible for triggering the preovulatory surge in LH and producing estrous behavior. Sexual receptivity lasts 1 to 4 days, with older animals in estrus longer. In most animals, ovulation occurs 36 to 44 hours after onset of estrus. Some of this variability in duration of the ovulatory phase can be explained by duration of estrus. Regardless of length of standing heat, sows ovulate two thirds to three fourths of the way through estrus. A sow in heat for 24 hours would be expected to ovulate 16 to 18 hours after onset of estrus. A sow that stands for 72 hours would be expected to ovulate 48 to 54 hours after onset of estrus. Natural ovulation in sows, determined using transrectal real-time ultrasound evaluation, lasts about 2 hours.5 Other studies have determined the duration to be up to 9 hours. Duration may be affected by technique and by mating, which advances ovulation.6
The role of follicle-stimulating hormone (FSH) during the ovulatory phase has been less well characterized than that of LH. A surge in FSH coincident with the LH surge has been reported, but the postovulatory surge in FSH is more distinct and appears to be responsible for activating small follicles for potential ovulatory growth during later cycles. Circulating FSH is inversely related to concentrations of inhibin, a follicular protein known to exert negative feedback on FSH. Inhibin is produced increasingly as follicles reach ovulatory size.
PREGNANCY
Embryo Survival and Development
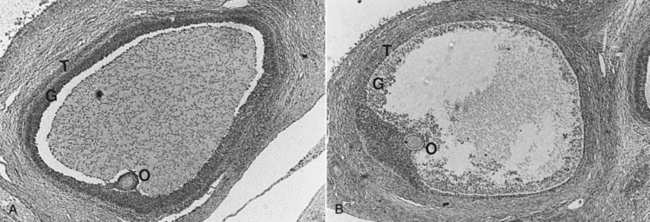
It generally is agreed that for sows when successful fertilization occurs, nearly 100% success in fertilization is achieved in swine. Between 20% and 30% of potential embryos (as reflected by number of corpora lutea) die during the first 30 days of gestation, and another 10% die before term. It appears that in most instances, embryo death occurs between days 13 and 20.7 Some proportion of embryo deaths is due to chromosomal aberrations and congenital lethal defects that are unavoidable. Porcine embryos migrate into the uterine lumen from the oviduct at about day 4, and growth is supported by uterine histotrophs. Embryos begin to secrete estradiol at approximately days 10 and 11 of embryonic life, coincident with rapid development from the spherical to the filamentous stage (Fig. 98-4). A second increase in estradiol occurs after day 14. The estradiol secretion is associated with specific uterine protein secretion, uterine epithelial cell morphology and secretory patterns, maternal recognition of pregnancy, and embryonic migration.7 Embryo size at this transitional stage of development can vary. Recent information indicates that strains of pigs with greater litter size, such as miniature swine selected for the D haplotype and Chinese Meishan swine, have blastocysts that grow more slowly and secrete estradiol more gradually than do domestic swine. As a result, intrauterine competition for space may be reduced during the critical period of distribution and elongation of embryos. Another hypothesis for variation in embryo development is that the later ovulations give rise to smaller embryos, and that initiation of estradiol production by older blastocysts inhibits this maturational process in the younger embryos. This hypothesis was not supported by research results showing that embryonic diversity at 98 hours was not related to duration of ovulation.5
Few therapies are available that reliably increase embryonic survival. Maintaining sows in a cool, calm environment for several hours after breeding seems to increase embryo survival. Limiting feed intake to a maintenance level for 2 to 5 days after breeding also has been suggested to be beneficial to embryo survival, presumably because of the relationship of intake with circulating progesterone levels.8 This effect is seen more often in gilts than in sows. Parity 1 and sometimes parity 2 sows typically have a reduced subsequent litter size. A practice of mating these females at their second postweaning estrus rather than the first has been shown to increase litter size dramatically, and embryo survival has been proposed as the mechanism. A high level of hygiene and ensuring that matings occur only during sexual receptivity will enhance efficiency of reproduction and could be partly mediated through embryo survival.
Uterine Capacity
Although ovulation rate places an absolute limit on litter size, embryo and fetal survival depend on the complex uterine factors described. A practical term for these collective uterine influences is uterine capacity. Several investigations have proved the importance of uterine capacity when ovulation rate is not limiting. For example, the ovulation rate and number of fetuses are correlated linearly up to a certain point, but then the magnitude of this association levels off, being described by most researchers as a quadratic relationship. When ovulation rate is greater than uterine capacity, increased embryo or fetal death (calculated as a percentage of the ovulations) occurs. An increase of 3.7 eggs was realized from nine generations of selection for ovulation rate, but only 20% of the increase was reflected in an increase in litter size.9 Direct selection for litter size for eight generations in the high ovulating line resulted in an increase of 1.06 in number of piglets, presumably as a result of enhanced uterine capacity. Uterine capacity may involve several mechanisms including physical size, degree of folding between uterine and placental tissues, and vascularity of the placenta.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree