CHAPTER 30 Clinical Reproductive Physiology and Endocrinology of Bulls
The bull exerts major influences on herd fertility and production whether he is bred with many females, using assisted reproductive technologies, or with relatively few via natural service. Despite this, relatively little selection pressure for reproductive traits has been placed on most bull populations. Furthermore, multiple-sire breeding, as practiced routinely by commercial cattle producers, makes it difficult to identify subfertile bulls. For example, it is estimated that 20% to 40% of unselected beef bulls in North America are subfertile.1 Because of this, there is increasing demand for breeding soundness evaluation, particularly for bulls destined for natural service. Knowledge of the anatomy, physiology, and behavior of bulls is necessary for veterinarians to conduct an adequate breeding soundness evaluation, investigate reproductive problems, and advise on reproductive management.
PRENATAL DEVELOPMENT
During embryogenesis, the gonad first arises from the mesonephros as undifferentiated tissue, which has the capability to subsequently develop in either male or female form. The precursors to the male reproductive tract system (i.e., the wolffian duct system) and the female tract (the müllerian duct system) are both present. Subsequent sexual differentiation is decided, in mammals, by the presence or absence of the Y chromosome, with females being XX and males XY. The presence of a Y chromosome results in male development, regardless of the number of X chromosomes present. Thus, the Y chromosome must contain the dominant inducer of testis formation; the testis determining gene (TDF or SRY). SRY is activated early in embryogenesis to commit the undifferentiated genital ridge to the testicular pathway. The early testis produces both testosterone (T; from the Leydig cells) and müllerian inhibiting substance (MIS; from the Sertoli cells). The latter induces the müllerian ducts to regress. Subsequent hormonal production induces male sexual differentiation. In the bovine, the differentiating gonad may be identified as a testis by 41 days after conception, with testosterone production (from fetal Leydig cells) evident soon thereafter.2 By 3 to 4 months following conception, the testes have generally passed through the inguinal canal and entered the scrotum, which is derived from the urogenital folds. Although the basic components of a functional male gonad are present at birth, the spermatogonia do not undergo meiosis until the onset of spermatogenesis at puberty. Thus, the basic structure of the testis (seminiferous cords and interstitial tissues) remains much the same from early fetal life until the onset of puberty.
REPRODUCTIVE ANATOMY
The reproductive organs of the mature bull include paired testes, each with a spermatic cord, an epididymis, and a deferent duct (ductus deferens), which culminates in an ampulla.3 In addition are paired vesicular glands, a prostate gland, paired bulbourethral (Cowper’s) glands, and a fibroelastic penis, which incorporates a sigmoid flexure (Fig. 30-1).4 The vesicular, bulbourethral, and prostate glands are often referred to as the accessory sex glands.
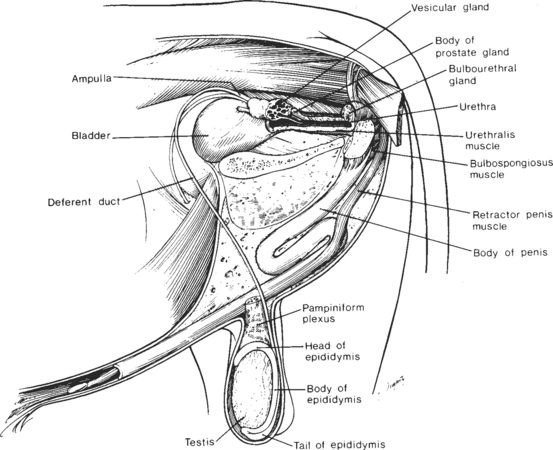
Fig. 30-1 Reproductive organs of the bull.
(From Amann RP: How a bull works. Proceedings of the 11th Technical Conference on A. I. and Reproduction (NAAB), 1986, p 7.)
The testes are suspended within the scrotum, a feature that is important for testicular thermoregulation, as discussed subsequently. Within the testis, most (70% to 90% by weight) of the parenchyma is composed of seminiferous tubules (Sertoli cells and layers of germ cells). The remainder consists of interstitial tissue (Leydig cells, blood and lymph vessels, and connective tissue). The mediastinum, an area of connective tissue extending lengthwise in mid-testis, contains blood vessels and tubules of the rete testis. The testicular parenchyma is encased in a thick, connective tissue capsule (tunica albuginea), which is, in turn, covered by a thin, serous membrane (tunica vaginalis propria).3
In general, the spermatogenic efficiency of healthy testicular parenchyma in bulls is remarkably consistent (approximately 10–12 × 106 spermatozoa per gram daily). As testes weight is highly correlated with scrotal circumference (r = 0.91 to 0.98 in young beef bulls1), this highly repeatable measure has gained wide acceptance as an estimate of sperm-producing capability, especially as testicular size is heritable in beef bulls (h2 ∼ 0.5).1 Although breed and environment may also influence testicular development, most variation in daily sperm production can be attributed to testicular size (represented by scrotal circumference), at least in younger (<4–5 years old) bulls.
Sertoli cells are amorphous, nucleated somatic cells that span the seminiferous epithelium and play a critical role in supporting and controlling germ cell development.4 Specialized junctions between adjacent Sertoli cells form the blood-testis barrier, dividing the seminiferous epithelium into basal and adluminal compartments.5 Spermatogonia lie between Sertoli cells and the tubule basement membrane, and other germ cells are located either in cytoplasmic crypts within Sertoli cells or sandwiched between adjacent Sertoli cells.
At birth, the bull penis is short and slender and lacks a sigmoid flexure, and its apex is fused to the inner lining of the prepuce. With time (and under the influence of androgens), penile and preputial tissues separate, the penis elongates, and a sigmoid flexure develops. Tissue separation proceeds irregularly and in many bulls is completed only after the onset of erectile activity. Thereafter, incomplete separation is defined as a persistent penile frenulum (otherwise known as a persistent raphe or tied penis). This condition, most commonly present in Angus, Beef Shorthorn, Hereford, Polled Hereford, and Beefmaster bulls, probably has a genetic basis in many cases.6 Although this condition is usually correctable with minor surgery, the consequences of perpetuating a genetic defect should be considered when this is done.
The prepuce is a double invagination of skin, with its internal lining everting upon penile erection to constitute much of the penile surface.3 A fan-shaped protractor prepuce muscle raises and lowers the distal portion of the prepuce and also controls the size of the preputial opening. Retraction of the membrane lining the inner prepuce is under the control of the retractor prepuce muscle. Lack of development of this muscle (a condition genetically linked with the polled gene in bulls), predisposes to chronic eversion of this membrane with increased risk of traumatic injury.
EPIDIDYMAL FUNCTIONS
The epididymis is far more than a passive organ, with functions including both sperm transport and maturation. Spermatozoa leaving the testis lack both the ability to survive in the female tract and to achieve unassisted fertilization. These capabilities are acquired in the epididymis. Other epididymal functions include the (1) energy-efficient storage of sperm while maintaining sperm fertility; (2) intermixing of recently formed and older spermatozoa to provide a temporal spectrum of optimal sperm function, and (3) changing sperm attributes and environment to permit survival and ensure fertilizing capability within the female tract.7
Sperm maturation occurs within the caput and corpus of the epididymis with this process requiring the coordinated secretion of specialized enzymes and proteins. During this process, changes occur in the sperm DNA-protein complex, plasma membrane, mitochondria, axonemal complex, plasma and acrosomal membranes, and sperm surface characteristics.7
SCROTAL TESTICULAR THERMOREGULATION
Scrotal testicular thermoregulation has been recently reviewed.8 Testicular temperature in bulls must be 2 to 6° C cooler than core body temperature for effective production of fertile spermatozoa. Several mechanisms act to regulate the testicular temperature. Of major importance is the pampiniform plexus, a complex venous network that surrounds the highly coiled testicular artery within the neck of the scrotum. This entire structure (vein and artery) is properly called the testicular vascular cone.8 The cone functions as a countercurrent heat exchanger whereby heat is transferred from arterial to venous blood. Scrotal skin is thin and relatively hairless, with extensive blood vessels that can dilate to increase heat loss. The testes and scrotum have complementary temperature gradients that result in a nearly uniform intratesticular temperature.8 Other mechanisms that help to cool the testes include relaxation of scrotal muscles, scrotal sweating, and whole-body responses (e.g., panting and peripheral vasodilation). Spermatogenesis occurs in an environment that verges on hypoxia. When testicular temperature increases, spermatogenic metabolism increases faster than does blood flow, and hence the testes become hypoxic.8 Therefore, testicular function is very susceptible to temperature increases due to either endogenous or exogenous factors (e.g., fever or high ambient temperatures, respectively). Increases in testicular temperature cause increased production of defective spermatozoa. The proportion of defective spermatozoa and the time required for recovery depend upon the nature and duration of the thermal insult.8 Severe insult may cause irreversible spermatogenic damage.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


