CHAPTER 7 Clinical Reproductive Anatomy and Physiology of the Mare
The dynamic functions of the ovaries, uterus, embryo, and fetus are beginning to be fully appreciated as mares are evaluated during different stages of the estrous cycle and pregnancy and during various seasons. Anomalies, injuries and age-related changes may occur in mares, necessitating the use of diagnostic, prognostic, and therapeutic procedures, which require a thorough knowledge of equine anatomy. Several references, including the corresponding chapter in the first edition of this text, are currently available and provide a comprehensive discussion of reproduction in the mare, complementing the information presented in this text.1–4
REPRODUCTIVE ANATOMY
The organs involved in reproductive function are not only physiologically but also morphologically dynamic. The reproductive tract in the mare consists of the ovaries, uterine tubes, uterine horns, uterine body, cervix, vagina, vestibule, and vulva. These are the organs that produce the oocyte, facilitate its fertilization, provide an environment for embryonic and fetal development, and transport the fetus from the maternal to the external environment. They cannot perform these functions without interacting with other organ systems. The normal development of the reproductive tract of the mare, as well as the physiology of the mare’s estrous cycle (upon which there are seasonal influences), depend on the neural and endocrine functions of the epiphysis (pineal gland) and hypothalamus (including the hypophysis or pituitary gland). The interactions of the epiphysis and hypophysis with other structures within the nervous and endocrine systems, the reproductive tract, and the mammary glands affect sexual behavior, pregnancy, foaling, and lactation.4 Anatomy textbooks by Sisson and Grossman; Nickel, Schummer, and Seiferle; and Dyce, Sack, and Wensing are the foundations of the anatomic knowledge of many veterinarians.5–7 Every effort will be made to remain consistent with their descriptions as well as with the nomenclature approved by the Nomina Anatomica Veterinaria (NAV) and the Illustrated Veterinary Anatomical Nomenclature.8,9 Unless specifically noted, all anatomic discussions will pertain to the postpuberal female.
The Reproductive Tract of the Mare
Suspensory Ligaments
Structure and function.
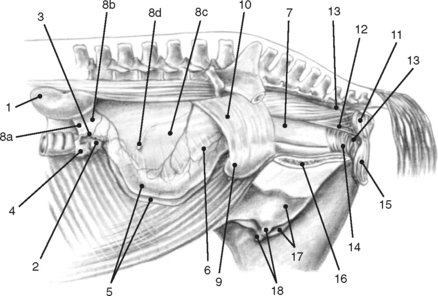
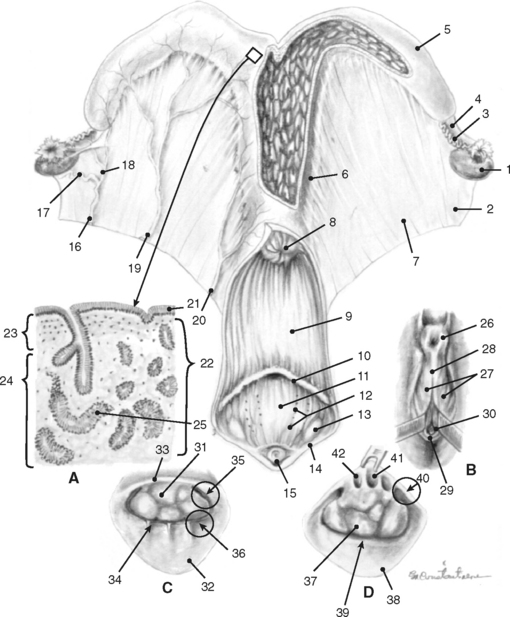
Bilaterally the ovaries, uterine tubes, and uterus are suspended from the lateral sublumbar and pelvic walls by the double-layered peritoneal folds referred to as the broad ligaments, divided into three anatomically nondelineated portions: the mesovarium (attached to the ovary), mesosalpinx (arising from the lateral surface of the mesovarium), and mesometrium (attached to the uterus and a portion of the cranial vagina) (Figs. 7-1 to 7-3).1,4–7,10 The cranial or free border of the mesovarium is the suspensory ligament of the ovary, which attaches in the sublumbar region.8,9,11 The ovarian bursa is a duplication of the broad ligament between the ovary and the uterine horn.4
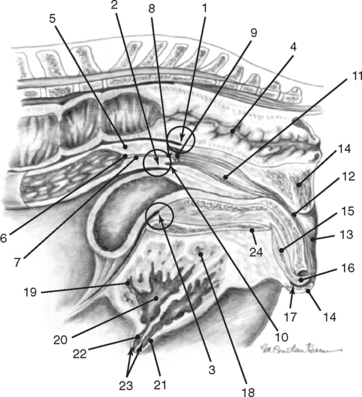
The broad ligaments allow for functional as well as physical attachment of the reproductive tract to the rest of the body. They provide an avenue for arteries, veins, lymphatic vessels, and nerves, which supply the ovaries, uterine tubes, and uterus. These ligaments also contain loose adipose and collagenous tissues as well as smooth muscle that is continuous with the outer longitudinal muscle layer in the uterine tube and uterus. The broad ligaments are also dynamic structures. During gestation, the vessels and smooth muscle mass enlarge in the broad ligaments, resulting in an apparent thickening. This suspensory apparatus allows for movement of the ovaries, uterine tubes, and uterus. Embryologically, peritoneal pouches form between the suspensory ligaments of the rectum, uterus, and urinary bladder and the roof and the floor of the pelvic cavity, respectively. Cranially, these pouches open into the peritoneal cavity, and they end caudally in cul-de-sacs in the pelvic cavity against the pelvic diaphragm. The rectogenital pouch is formed by reflection of the peritoneum from the rectum onto the vagina. The reflection of peritoneum from the urinary bladder onto the vagina forms the vesicogenital pouch (Fig. 7-2).1,4,7–11
Clinical aspects.
The anatomy of the broad ligaments can be clinically relevant in several situations. Exterior ization of the uterine horns or ovaries during surgical procedures is limited by the suspensory ligaments. The mesovarium is located for écrasement and ligation during ovariectomy, and in standing animals, local anesthetics are applied to the mesovarium prior to performing these procedures (Figs. 7-1 and 7-3).12 As the vagina is rarely involved in uterine torsion in the mare (in contrast to the cow), transrectal palpation of the broad ligaments is necessary for diagnosis of uterine torsion and determination of the direction of torsion. In cases of uterine and ovarian arterial rupture, the mesometrium may contain the hemorrhage, or bleeding may dissect the broad ligament resulting in its rupture and exsanguination of the mare. Hematomas can be found in the mesometrium during routine postpartum examinations, and they may or may not be associated with clinical signs. Rupture of hematomas several weeks following parturition has been recorded.4 It is important to remember that the anatomic boundaries of the rectogenital and vesicogenital pouches are variable and that this anatomic variability can be of clinical significance when performing an ovariectomy via a vaginal approach (colpotomy) or in assessing whether contamination of the peritoneal cavity has occurred following a vaginal or rectal injury (Fig. 7-2).1,4,5,12
The Ovaries
As in other domestic mammals, the ovaries perform exocrine (gametogenic) and endocrine (hormone production) functions in the mare. Exocrine function of the ovaries justifies the existence of the tubular genitalia and allows for the ovaries’ hormonal integration and control of the reproductive tract.1,3,4
Location.
The ovaries are traditionally the landmarks for transrectal palpation of the reproductive tract in mares. The ovaries and uterine tubes are the most cranial structures of the reproductive tract and may be found as far cranially as the third lumbar vertebra or as far caudally as the fifth lumbar vertebra. They are not as freely moveable as cows’ ovaries, and, in nonpregnant animals or very early in gestation, they are often located 5 to 10 cm directly cranial to the upper third of the ipsilateral ileal shaft in the sublumbar region. The kidney lies cranial to the ipsilateral ovary by a distance ranging from 5 to 30 cm with the left kidney generally closer to the corresponding ovary. The left ovary is normally situated 2 to 3 cm further caudad than the right. The ovaries may be in contact with the corresponding uterine horn or may be separated by a distance of up to 5 cm. In circumstances in which the uterine horn is readily located, this anatomic relationship may be helpful in locating the ipsilateral ovary (see Figs. 7-1 and 7-3). As discussed previously, the mesovarium allows for variable positions of the ovaries depending on the stage of estrous cycle or pregnancy, seasonal influences, and the location of other abdominal organs. During pregnancy, the ovaries are frequently found pulled cranioventrally and medially. The ovaries frequently lie on top of the intestinal mass and are in contact with the dorsal abdominal wall; however, they may be found among the adjacent viscera.1,4,5
External structure.
The mare’s ovaries are reniform in shape and, in mares of light breeds, range from 5 to 8 cm in length, 2 to 5 cm in width, and 3 to 5 cm in height during the ovulatory season. Ovarian weight varies with age and stage of the estrous cycle, ranging from 30 to 120 g per ovary. The ventral (free) border of the ovary is concave and is indented by the ovulation fossa (ovarian fossa; NAV), which is an anatomic structure specific for the mare (Fig. 7-3).1,3,4 When the intestinal viscera are removed and the reproductive tract is suspended by the broad ligaments, the ovaries are described as having medial and lateral surfaces, dorsal and ventral borders, and cranial (tubal) and caudal (uterine) extremities or poles. The dorsal (attached) border is convex, and, since this is where the mesovarium attaches and where vessels and nerves enter the ovary, it is correctly termed the hilus of the ovary. The ventral (free) border of the ovary is concave and is indented by the ovulation fossa (Fig. 7-3).1,4,5,8–11 The caudal or uterine extremity of the ovary is attached to the uterus near the tip of the uterine horn by the proper ligament of the ovary (round ligament of the ovary or utero-ovarian ligament), which is a band of smooth muscle within the broad ligament (Figs. 7-1 to 7-3). In the embryo, this structure is continuous with the round ligament of the uterus and is homologous to the proper ligament of the testis in the male (part of the remnant of the gubernaculum testis).1,4–6,8–10 Additional details regarding the histology of the external surfaces of the ovary are covered in the corresponding chapter of the first edition of this text.4
Internal structure and function.
In most domestic mammals the ovaries consist of a peripheral parenchymatous zone (cortex), containing various stages of follicular and luteal gland development, surrounding a central vascular zone (medulla), comprising collagenous connective tissue rich in blood vessels. In the mare, the central parenchymatous zone, which surfaces at the ovulation fossa, is surrounded by the vascular zone.1,4–7,11 Development of this unusual arrangement of the zona parenchymatosa and zona vasculosa in the mare will be discussed later.
The primary functions of the ovary are oogenesis (production of female gametes, oocytes, or ova) and steroidogenesis (production of estradiol and progesterone). During early gestation, the primordial germ cells arise from the epithelium of the embryonic yolk sac and migrate through the developing mesentery to the gonadal ridge (ovarian anlage) in its position contiguous with the mesonephros. Mitosis and meiosis of the oogonia do not occur beyond the sixth month of gestation and do not commence again until after puberty. There is substantial atresia of oocytes during fetal life, with only a portion surviving to parturition.1,4,7
The structural and functional unit of the ovary is the follicle. A primary oocyte surrounded by a single, flattened cell layer is a primordial follicle. There is an intact basal lamina separating the single layer of what will become granulosa cells from the adjacent stromal tissue, which eventually develops into the theca folliculi (theca cells). Mares are born with a finite pool of primordial follicles. It has been estimated that there is a reserve pool of approximately 36,000 primordial follicles in the mare (in contrast to over 120,000 primordial follicles in the cow). At any time during the ovulatory season, 100 (versus 300 in the cow) primordial follicles have left the reserve pool and have entered the active pool of follicles and are undergoing growth and differentiation (folliculogenesis). At this point in their development, the follicles are called primary follicles. Once a primordial follicle joins this pool, it is destined to become atretic or ovulate. The oocyte grows in size and the zona pellucida is formed. The cells surrounding the oocyte differentiate into granulosa cells. A primary follicle is transformed to a secondary follicle when the single layer of granulosa cells differentiates into several layers. Primary and secondary follicles have traditionally been described as preantral follicles.1,4
Fluid exuded from granulosa cells of secondary follicles coalesces to form an antrum, and the follicle is transformed into a tertiary or antral follicle, which is primarily estrogenic. The wall of the follicle is formed by granulosa and theca cells. In the mare, follicles approximately 0.3 mm in diameter begin to form antrums, and these antrums may reach a size of 35 to 50 mm with follicular walls 5 to 6 mm in thickness. Studies in mares have estimated that it may take as long as two estrous cycles (42 days) for a follicle to grow from 0.1 mm to l mm. Other research has suggested that in cows the period required for a primary follicle to develop into a preovulatory follicle may be as long as 60 days. In mares, 50 to 75% of follicles greater than 1 mm in diameter present at any time are undergoing atresia, which is irreversible. Atretic follicles demonstrate degenerative changes in the oocyte and follicular epithelium. Fibroblastic remodeling of the remnants of large follicles results in scar tissue (corpus fibrosum atreticum).1,4
During each estrous cycle there may be up to two waves of follicular growth during which several follicles attain diameters greater than 15 mm. Usually one follicle ovulates from the primary wave, but occasionally there can be multiple ovulations or ovulation(s) from the secondary wave, as well.1,3 The small number of follicles that ovulate migrate toward the ovarian fossa as their diameter increases, and they soften as they get closer to evacuation. Granulosa cells cease to divide a few days prior to release of the ovum. Immediately prior to follicle evacuation, granulosa cells undergo further differentiation, theca cells begin to degenerate, and there is a thinning of the follicular wall toward the exclusive site of ovulation in the mare, the ovulation fossa (Fig. 7-3).1,3–6 At the time of ovulation, the first meiotic division is complete, and a secondary oocyte (arrested in metaphase II) is released.
A short time following ovulation, folds of stromal tissue that are accompanied by distended blood vessels, are formed by the collapsed inner wall of the follicle. Within 24 hours, granulosa cells have begun to undergo further growth and differentiation and are beginning to secrete progesterone. These changes to the granulosa cells constitute the process commonly referred to as luteinization. Proliferating capillaries accompanied by hypertrophied fibroblasts intermingle with luteinizing granulosa cells and replace degenerating theca cells. Nine days after the formation of the luteal gland, two types of luteal cells are evident, large and small. Eighty-five percent of the luteal cell population is composed of large luteal cells, which are almost four times larger than they were at the time of ovulation. The small cells are not derived from theca cells, as in other species, but are most likely granulosa cells in transition to becoming large luteal cells. By the twelfth day of the luteal phase in nonpregnant mares, the large luteal cells have decreased in size and in number. At the time of the next ovulation following luteal regression, the large luteal cells have undergone degeneration and the corpus albicans has been formed.1,4
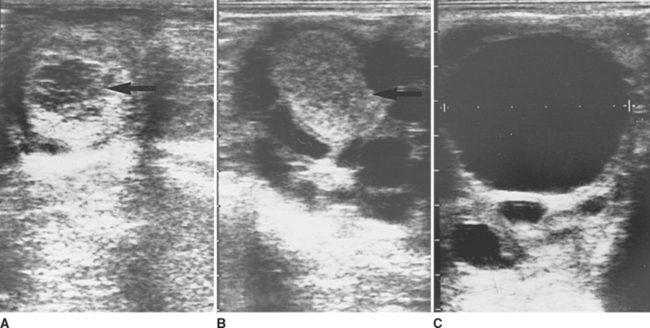
Luteal glands have two distinct appearances when examined by ultrasonography (Fig. 7-4). Approximately 50% of the luteal structures contain a central blood clot that occupies greater than 10% of the cross-sectional area and progress through a corpus hemorrhagicum stage during formation of the mature corpus luteum (CL; Fig. 7-4, A). The central clots remain during the life of the CL but decrease in size and become more organized. The other 50% of ovulations result in formation of a solid or uniformly echogenic CL (Fig. 7-4, B). There is no significant difference in progesterone secretion by these two differently appearing luteal structures.1,3,4 In nonpregnant mares, the luteal gland begins to regress approximately 12 to 14 days following ovulation and begins to form a corpus albicans by the start of the following estrus.1,4
Prepuberal ovarian development.
At birth the ovaries are oval rather than reniform in shape; cuboidal germinal epithelial cells cover their ventral (free) surface, and the ovarian fossa has not yet formed. The fetal gonads at their greatest size during gestation are ten times larger than the foal’s ovaries at parturition. By 3 months of age, the cortex (parenchymatous zone) is located beneath the infundibulum suggesting the future site of ovulation. The parenchymatous zone becomes invaginated into the vascular zone causing the ovarian poles to be drawn toward each other. In this manner, the unique anatomic relationship between the zona parenchymatosa and zona vasculosa seen in the ovary of the postpubertal mare begins to develop. As early as 5 to 7 months of age and certainly by puberty, the ovaries are kidney-shaped with a definite ovulation fossa.1,4,11
Clinical aspects.
Transrectal examination of the ovaries by palpation and ultrasonography is one of the more commonly performed diagnostic procedures in mares. Confidence in locating the ovaries and identifying the normal, pertinent structures allows efficient determination of the mare’s suitability for breeding and an accurate diagnosis of ovarian pathology, as well as a decreased risk of rectal injuries. Larger follicles can frequently be palpated as fluctuant structures; however, as the mare always ovulates from the ovulation fossa, smaller preovulatory follicles may sometimes be difficult to assess by transrectal palpation alone.3,13–15 This certainly may be the case in younger or smaller mares that exhibit some resistance to the procedure. Although recent ovulations can be appreciated by palpation, luteal structures are often difficult to detect, especially if the mare’s estrous cycle has not been followed closely by serial palpations. Transrectal ultrasonic imaging of the mare’s ovaries allows visualization of follicles, ovulations (during estrus and diestrus), ovarian hematomas, and CLs (Fig. 7-4). In addition, ultrasonography has made possible the detection of multiple ovulations, anovulatory hemorrhagic structures, ovarian abscesses, and tumors.1,3,4,13–16 Using ultrasonography, it has been documented that there is an increased incidence of left-sided ovulations in maiden mares and that there is no predilection for nocturnal ovulations, as was previously thought.1 The routine use of cooled, transported, and frozen semen and the performance of embryo and oocyte collection and transfer procedures has necessitated refinement of the ability to pinpoint the time of ovulation using ultrasonic imaging of the ovaries.4,17–19 Irregularly shaped or flattened follicles with echogenic spots in the follicular fluid, as well as decreased follicular pressure and separation and hyperechogenicity of the follicular wall, are some of the changes noted just prior to ovulation.3,4,15,20
Knowledge of normal ovarian morphology and development can be helpful in the diagnosis of a variety of pathologic conditions and is critical for safe and successful oocyte recovery.1,3,4,17–19 Not infrequently, mares are palpated, and both ovaries are found to be small and inactive. Seasonal influences account for the majority of these cases, but a history of anabolic steroid administration or chromosomal abnormalities (leading to gonadal dysgenesis) can account for some of these cases.4,13,14 Although subject to some debate, it is generally thought that mares do not experience cystic follicular degeneration, as do other species.4,13,16 Large ovarian follicles, which might be mistaken for follicular cysts, are often found during the early spring or late fall due to seasonal influences. Mares may develop epithelial inclusion cysts (fossa cysts), which can invade the ovarian stroma, and these cystic structures can potentially be a cause of infertility in older mares. Older mares are also more likely to have abnormal ovulations, which result in defective oocytes and embryos.4,13–16,21 Ovarian hematomas are frequent occurrences, and although the ovary may be greatly enlarged, the mare continues normal ovarian activity. A number of different types of ovarian tumors have been observed in mares, including granulosa-theca cell tumors, dysgerminomas, teratomas, and cystadenomas.1,3,4,14,16,21 The most common of these is the granulosa or granulosa-theca cell tumor. These tumors can be very large, and affected mares can exhibit several different patterns of abnormal behavior. The contralateral ovary is usually small and inactive, and the ovulation fossa is normally not palpable. However, exceptions have been observed, and these cases may represent the clinical presentation early in the course of tumor development.4 It has been reported that excessive adrenocortical tissue can occur on the surface of the ovary and invade adjacent tissues. This most likely represents a pathologic manifestation of the adrenocortical nodules frequently seen in mares.4,16
The Uterine Tubes
The uterine tubes are paired structures, each associated with an ovary and uterine horn. Known by a variety of names including oviducts, salpinges, and fallopian tubes, these structures are the initial segment of the tubular genital tract. Like the uterus and vagina, they are derived embryologically from the paramesonephric ducts (müllerian ducts) and perform a variety of functions.1,4,7
External structure and location.
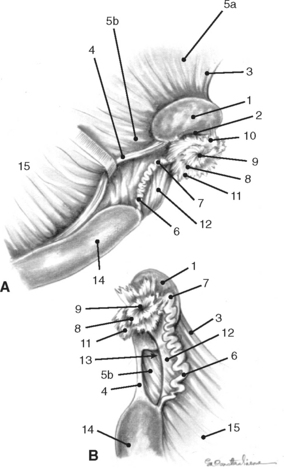
The external surface of the uterine tube is covered by visceral peritoneum or serosa that is an extension of the squamous epithelium (mesothelium) of the mesosalpinx. This epithelium is contiguous with the mucosal lining of the infundibular portion of the uterine tube.1,4 When dissected free from the mesosalpinx, each uterine tube, which is located adjacent to the lateral surface of the ipsilateral ovary, is 20 to 30 cm in length and is divided into three segments—the infundibulum, the ampulla, and the isthmus (Figs. 7-1, 7-3, and 7-5).The funnel-shaped infundibulum has irregular fimbriae along its margins and a centrally located abdominal ostium. The tortuous ampulla, which is of a similar diameter to the abdominal ostium of the infundibulum, gradually decreases in diameter and extends from the infundibulum to a poorly defined point in proximity to the lateral surface of the uterine extremity of the ovary, where the isthmus begins. The isthmus is less tortuous, and its terminus, the uterotubal (tubouterine) junction, consists of a small papilla projecting into the uterine lumen. The oviductal lumen communicates with the lumen of the uterine horn through the uterine ostium in the papilla.1,4,7–9,11
Internal structure and function.
There is a divergence in the internal structure of the portions of the oviduct and this structural alteration suggests that the primary functions of the uterine tube vary from the ovarian to the uterine extremities. The muscular layer (myosalpinx or tunica muscularis) consists primarily of an inner layer of circularly arranged muscle fibers covered by an outer layer of longitudinally oriented fibers. These longitudinally arranged fibers are continuous with the smooth muscle present in the mesosalpinx. There are very few muscle fibers in the fimbriae, and the tunica muscularis of the ampulla is thin. The muscular layer of the isthmus, however, is very well developed. A sphincter of circular smooth muscle is formed at the uterotubal junction.1,4
The mucosa of the infundibulum is highly folded longitudinally and covered by columnar epithelium, which is frequently ciliated. Changes in the estrous cycle are reflected by alterations in ciliogenesis and ciliary action. The mucosa of the ampulla is extremely plicated with primary, secondary, and even tertiary folds. This complexity of folding is more obvious in the mare than in ruminants, but the mucosa of the isthmus has much less folding than that of ampulla.1,4,7
The oocyte with its cumulus of granulosa cells is swept into the infundibulum by cilia. Very little, if any, follicular fluid enters the uterine tube following ovulation. The proximal portions of the oviducts provide a conduit for transport of the oocyte to the site of fertilization at the junction of the ampulla and the isthmus. The uterotubal junction allows spermatozoa to enter the isthmus following breeding, yet there is no reflux of uterine contaminants into the oviduct. The isthmus provides sites for capacitation and storage of spermatozoa while they await an oocyte to fertilize. The nonciliated epithelium of the uterine tube is predominantly secretory in nature and produces proteins and glycosaminoglycans. These secretory products may play a role in mediating capacitation of spermatozoa, fertilization of the ovum, and early embryonic cleavage. Preovulatory follicular fluid has a role in regulating oviductal protein production in vitro and in vivo (uterine tube ipsilateral to ovulation).4 The mare is unique among domestic mammals in that only fertilized oocytes (developing embryos) enter the uterus approximately 6 days following ovulation. Unfertilized ova are retained in the middle third of the uterine tube for several estrous cycles among collagenous material that may contain cells similar to fibroblasts. Some have suggested this selective transport of fertilized oocytes is the initial step in “maternal recognition of pregnancy” in the mare.1,4,5
Clinical aspects.
The uterine tube is the one portion of the reproductive tract of mares where functional abnormalities or pathologic changes and their significance are less likely to be appreciated. The myoelectrical activity of the myosalpinx can be influenced by oxytocin and prostaglandin F2α (PGF). The clinical relevance of these modifications in oviductal function is yet to be determined. The presence of debris in the uterine tube has been described and is hypothesized to have an effect on tubal patency. Several techniques have been developed for the assessment of oviductal patency, but controversy exists over the prevalence of nonpatent oviducts and whether the observed debris actually represents a pathologic change.4,22 Hydrosalpinx is rare in mares, but cases have been reported.3,4,16,21 Mild to moderate inflammation and adhesions of the uterine tube have been noted in postmortem examinations of the horse; yet, as with other conditions observed in the uterine tubes, it is not known which oviductal changes have a negative effect on fertility and which represent normal variations. Several types of parovarian cystic structures occur in the vicinity of the oviducts. Some of these cysts are of mesonephric origin (epoöphoron and paroöphoron cysts). Although generally small and clinically significant, some of these structures, especially epoöphoron cysts, can become fairly large in the mare.4,13,16,21 Despite being listed as mesonephric in origin by some references, hydatids of Morgagni are, based on histologic evidence, paramesonephric in origin and represent accessory uterine tubes, which can become quite large and impede proper function of the reproductive tract.4,16,21
The Uterus
The uterus is a dynamic organ, which has several diverse functions. The uterus facilitates spermatozoal transport to the uterine tubes, and optimal embryonic and fetal development depend on an appropriate uterine environment. Fetal transport from the maternal to external milieu at the expected time requires coordination of activity between the uterus, fetus, and various neuroendocrine organs. Elimination of contaminants following breeding and rapid postpartum uterine involution require efficient uterine defense mechanisms. The cervix serves as a barrier to foreign materials during pregnancy and allows passage of a foal at parturition. Changes in ovarian steroid production or alterations in uterofetoplacental hormone metabolism can cause uterine anatomy and function to vary.1,4
External structure and location.
Embryologically derived from the paramesonephric ducts, the uterus of mares is bicornuate with a relatively long body. The uterus consists of paired uterine horns, a single body, and the cervix (see Figs. 7-1, 7-2, and 7-5). The cervix represents the thickened caudal portion of the uterus. The terminal segment of the uterine cervix (portio vaginalis) projects into the vaginal lumen. Externally, it is difficult to differentiate the uterine body from the cervix; however, the cervix can readily be distinguished by palpation. The surface of the uterus is covered by serosa or visceral peritoneum (perimetrium), which is continuous with the mesothelium in the broad ligaments. Viewed from above, the uterus is Y- or T-shaped. In nonpregnant mares, each uterine horn varies in length from 20 to 25 cm. In parous mares the horns may be asymmetrical. The uterine body is approximately 20 cm long. The cervix averages 5 to 7 cm in length and 3 to 4 cm in width. The intercornual ligament is much smaller and less distinct than in cattle. The uterus is capable of wide variations in size, shape, and location depending on the stage of the estrous cycle or pregnancy, seasonal influences, and degree of handling. The uterine horns are generally located entirely in the abdominal cavity. In the nonpregnant mare, the uterine body is found immediately in front of and frequently just ventral to the cranial brim of the pelvis. It can also be partially located in the pelvic cavity where it is continuous caudally with the cervix.1,4–7,10,11
Internal structure and function.
As discussed previously, at the tip of each uterine horn, there is a small papilla containing a sphincter of circularly arranged smooth muscle fibers where the uterine ostium of the oviduct is located (Fig. 7-5). The dynamic nature and functional significance of the uterotubal junction have already been discussed. Where the uterine horns join the body, there is a short median septum. This septum and the papillae of the uterotubal junctions are important structures to be identified during endoscopic examination of the uterus (hysteroscopy) or nonsurgical deposition of semen within the oviduct.1,4,12,13,22–24
In contrast to ruminants, there are no caruncles (permanent elevations) in the mare’s endometrium. The endometrial surface is composed of longitudinal folds that range in number from five to fifteen (Fig. 7-5). These endometrial folds in conjunction with the relatively flaccid uterine walls result in the existence of capillary spaces between adjacent folds rather than a central uterine lumen. It has been theorized that capillary action is partially responsible for the movement of secretions and inflammatory cells that collect in these spaces.1,4,7,10 It seems logical that similar factors may contribute to rapid transport of spermatozoa from the cervix to the uterotubal junction.
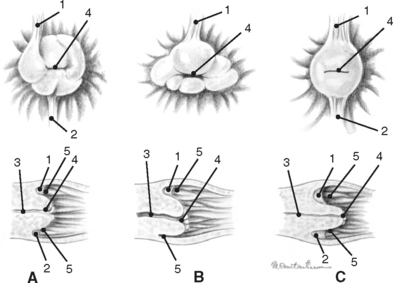
The internal uterine ostium is funnel-shaped and is the beginning of the cervical canal. The external uterine ostium is located in the intravaginal segment of the cervix (portio vaginalis). Longitudinally folded like the endometrium of the uterine body and horns, the mucous membrane lining the cervical canal is pale rather than the typical reddish brown (ranges from yellow to reddish brown) observed in the rest of the uterus. The circular folds (plicae circulares or annular rings) present in the cervical canal of ruminants are absent in the cervix of the mare. Folds of mucous membrane extend through the external uterine ostium. The dorsal and ventral folds may continue onto the vaginal wall to form frenulums dorsally and ventrally, respectively (Fig. 7-6).1,4,11–13
The uterine wall is divided into the perimetrium, myometrium, and endometrium. The myometrium is composed of an outer layer of longitudinally oriented muscle fibers, a middle vascular layer, and a thick inner layer of circular smooth muscle. The vascular and longitudinal muscle layers are continuous with the vasculature and musculature, respectively, in the mesometrium. The endometrium (mucosa) consists of simple columnar epithelium overlying a lamina propria (Fig. 7-5, Inset A). The height of the cells and the degree of ciliation is dependent on the stage of the estrous cycle. The endometrial folds are formed by aglandular, longitudinally arranged cores of connective tissue that are covered by mucosa. The lamina propria of the endometrium extends from the basement membrane of the epithelium to the inner layer of myometrium. There is no muscular tissue in the lamina propria, which consists of the 1-mm thick stratum compactum (high density of stellate, stromal cells) and the stratum spongiosum (low density of cells with abundant interstitial fluid and prominent tubular branched glands).4,25 These glands in the lamina propria are a distinguishing feature of the endometrium of the uterine body and horns. The glands open at the epithelial surface and extend to the depth of the lamina propria. Like the luminal epithelium, the lining of the endometrial glands consists of a single layer of columnar cells whose height is dependent on the stage of the estrous cycle and seasonal influences.1,4,25,26 With regards to the pre- and postnatal development of these endometrial glands, it has recently been shown that the final differentiation and maturation of the secretory portion of the endometrial glands occurs after the first ovulation and requires exposure to progesterone following estrogen priming.27 Gap junctions exist between adjacent cells in the luminal and glandular epithelium, and this feature of cells in the endometrium may allow for autocrine and paracrine functions of some hormones.4 Endometrial glands have varying degrees of tortuosity and patterns of distribution depending on ovarian steroid hormone production and the time of the year. Straightened glands and endometrial edema are typically seen during the follicular phase of the estrous cycle (estrus).1,25,26 During pregnancy, endometrial glandular secretions (histotrophs) and associated carrier proteins may play a role in establishing a normal uterine environment for embryonic and fetal development.28 The diffuse epitheliochorial placentation (microcotyledonary) observed in the mare reflects the absence of caruncles and underscores the importance of the entire epithelium in supporting fetal maturation.1,4,29
The endometrium plays an important role in the regulation of luteal function during the estrous cycle and pregnancy in the mare. The uterine mucosa is the source of the luteolysin PGF, and the embryo’s ability to prevent endometrial synthesis and release of PGF and facilitate “maternal recognition of pregnancy” will be discussed later in this chapter.1,4,29 In a process unique to the mare, involving metalloproteinases, chorionic girdle cells (fetal origin) initially attach to and then invade the luminal epithelium beginning at approximately day 35 of gestation. These trophoblastic cells engulf the epithelium, infiltrate the lamina propria, and differentiate into endometrial cups. Endometrial cups, which completely regress by the end of the fifth month of pregnancy due to a maternal cellular response, are specialized secretory structures that play a role in maintenance of the primary CL and the formation of supplementary CLs (also called secondary CLs by some authors) during gestation (second and third luteal response to pregnancy, respectively). The endometrial cups have a functional duration of 60 to 80 days that is independent of fetal viability. There may be a delay in return to normal ovarian and behavioral cyclicity following pregnancy termination if functional endometrial cups are present.1,4,29
Histologically, the structure of the uterine cervix reflects its function. There is a large number of mucigenous cells in the cervical epithelium. The cervix produces mucus, which serves as a lubricant or sealant depending on the stage of the estrous cycle or pregnancy. As compared to the uterine wall in the horns and body, there is an increased amount of collagen, as well as a prominent layer of circular smooth muscle containing many elastic fibers in the cervical region. The ability of the cervix to constrict and dilate is critical for successful breeding, pregnancy, and parturition.1,4
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree