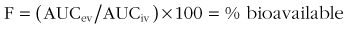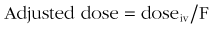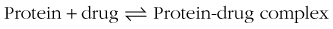Clinical Pharmacokinetics and Pharmacodynamics
Basic Information 
Procedure
• ADME: Mathematical evaluation of the rates of drug absorption (A), distribution throughout the body (D), along with metabolism (M), and ultimate excretion from the body (E).
 Indicates the process whereby drug is transferred from the site of administration to the systemic circulation.
Indicates the process whereby drug is transferred from the site of administration to the systemic circulation. Bioavailability (F) is the fraction of the dose administered extravascular that reaches intact the systemic circulation.
Bioavailability (F) is the fraction of the dose administered extravascular that reaches intact the systemic circulation. Determined by comparing the area under the plasma drug concentration curve versus time (AUC) after extravascular (EV) administration to the AUC after intravenous (IV) administration.
Determined by comparing the area under the plasma drug concentration curve versus time (AUC) after extravascular (EV) administration to the AUC after intravenous (IV) administration.  If the EV AUC is significantly less than the IV AUC, then the drug dose must be adjusted for the EV route.
If the EV AUC is significantly less than the IV AUC, then the drug dose must be adjusted for the EV route.  For IM or SC injections: Poor circulation at injection site, improper injection technique, insoluble complex formation
For IM or SC injections: Poor circulation at injection site, improper injection technique, insoluble complex formation If a drug has a narrow therapeutic margin and the F is low, with substantial variation between horses, then there may be no ideal dose for that formulation.
If a drug has a narrow therapeutic margin and the F is low, with substantial variation between horses, then there may be no ideal dose for that formulation. The oral F for enrofloxacin in horses is approximately 50%, so suggested oral doses are twice the IV doses.
The oral F for enrofloxacin in horses is approximately 50%, so suggested oral doses are twice the IV doses. The oral F for ciprofloxacin is approximately 6%, so it is not feasible to use oral ciprofloxacin in horses.
The oral F for ciprofloxacin is approximately 6%, so it is not feasible to use oral ciprofloxacin in horses. Determined by the drug’s ability to cross biologic membranes and reach tissues outside the systemic circulation.
Determined by the drug’s ability to cross biologic membranes and reach tissues outside the systemic circulation. Volume of distribution (Vd) (L/kg): The apparent volume of the body in which a drug is dissolved, but it does not correspond to any specific physiologic compartment.
Volume of distribution (Vd) (L/kg): The apparent volume of the body in which a drug is dissolved, but it does not correspond to any specific physiologic compartment. Vd is used to calculate the dose of drug given to produce a desired plasma drug concentration, (eg, a loading dose).
Vd is used to calculate the dose of drug given to produce a desired plasma drug concentration, (eg, a loading dose). Although Vd describes the extent of distribution of a drug, it does not confirm penetration of a drug to specific tissues.
Although Vd describes the extent of distribution of a drug, it does not confirm penetration of a drug to specific tissues. When penetration into specific tissues is unknown, a large Vd drug (Vd > plasma volume) is more likely to distribute there than a low Vd drug.
When penetration into specific tissues is unknown, a large Vd drug (Vd > plasma volume) is more likely to distribute there than a low Vd drug. Any condition that changes extracellular fluid volume affects the plasma concentrations of drugs with low Vd values (eg, competition with endogenous substances for protein binding sites).
Any condition that changes extracellular fluid volume affects the plasma concentrations of drugs with low Vd values (eg, competition with endogenous substances for protein binding sites). Aminoglycosides and nonsteroidal antiinflammatory drugs (NSAIDs) have low Vd values and plasma concentrations and risks of toxicity are increased in dehydrated horses.
Aminoglycosides and nonsteroidal antiinflammatory drugs (NSAIDs) have low Vd values and plasma concentrations and risks of toxicity are increased in dehydrated horses. Drugs with high Vds are not significantly affected by changes in body water status but may be affected by changes in body fat.
Drugs with high Vds are not significantly affected by changes in body water status but may be affected by changes in body fat. Moxidectin distributes into fat, therefore higher plasma concentrations and a greater risk of toxicity are seen in foals or debilitated adult horses.
Moxidectin distributes into fat, therefore higher plasma concentrations and a greater risk of toxicity are seen in foals or debilitated adult horses. If the protein binding is reversible, then a chemical equilibrium will exist between the bound and unbound states:
If the protein binding is reversible, then a chemical equilibrium will exist between the bound and unbound states:  Bound drug is too large to pass through biologic membranes, so only free drug is available for delivery to the tissues and to produce the desired pharmacologic action.
Bound drug is too large to pass through biologic membranes, so only free drug is available for delivery to the tissues and to produce the desired pharmacologic action. Bound drug may act as a drug reservoir or depot, from which the drug is slowly released as the unbound form.
Bound drug may act as a drug reservoir or depot, from which the drug is slowly released as the unbound form. Drug-drug interactions from protein displacement are rarely clinically significant and typically do not require dosage adjustment.
Drug-drug interactions from protein displacement are rarely clinically significant and typically do not require dosage adjustment. Increased anticoagulant effects seen with concurrent administration of phenylbutazone and warfarin are due to inhibition of hepatic metabolism of warfarin from phenylbutazone and not from displacement from protein binding sites.
Increased anticoagulant effects seen with concurrent administration of phenylbutazone and warfarin are due to inhibition of hepatic metabolism of warfarin from phenylbutazone and not from displacement from protein binding sites.• Lipid solubility and drug ionization (the pH-partition hypothesis)
 The degree of ionization for a weak acid or weak base depends on the pKa of the drug and the pH of the surrounding fluid.
The degree of ionization for a weak acid or weak base depends on the pKa of the drug and the pH of the surrounding fluid. When the pH is equal to the pKa of the drug, then the drug is 50% ionized and 50% nonionized (log 1 = 0).
When the pH is equal to the pKa of the drug, then the drug is 50% ionized and 50% nonionized (log 1 = 0). Basic drugs can be “ion trapped” when they move from the plasma to sites where fluids are more acidic than plasma such as cerebrospinal fluid, milk, and infected tissues.
Basic drugs can be “ion trapped” when they move from the plasma to sites where fluids are more acidic than plasma such as cerebrospinal fluid, milk, and infected tissues. Oral and injectable “long-acting “drug formulations are often slowly absorbed into the systemic circulation.
Oral and injectable “long-acting “drug formulations are often slowly absorbed into the systemic circulation. Flip-flop kinetics is identified by comparing the plasma concentration versus time curve for the extravascular route of administration to the curve after the drug is given intravenously. If the slopes of the elimination phases are not parallel, then absorption is limiting elimination (Figure 1).
Flip-flop kinetics is identified by comparing the plasma concentration versus time curve for the extravascular route of administration to the curve after the drug is given intravenously. If the slopes of the elimination phases are not parallel, then absorption is limiting elimination (Figure 1). Biotransformation or drug metabolism converts the drug in the body to a metabolite that is more readily excreted.
Biotransformation or drug metabolism converts the drug in the body to a metabolite that is more readily excreted. Other tissues such as the kidney, lung, small intestine, and skin also contain biotransformation enzymes.
Other tissues such as the kidney, lung, small intestine, and skin also contain biotransformation enzymes. Cl is the sum of all tissue clearances, where renal clearance (ClR) and hepatic clearance (ClH) are the major routes of elimination.
Cl is the sum of all tissue clearances, where renal clearance (ClR) and hepatic clearance (ClH) are the major routes of elimination. The body is considered a compartment of fluid with a definite volume (Vd) in which a drug is dissolved.
The body is considered a compartment of fluid with a definite volume (Vd) in which a drug is dissolved.Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree