Clinical Examination of the Female Reproductive Function
History
Medical and reproductive histories of the individual and the herd should be as complete as possible. Availability of detailed breeding and health records varies from one herd to another and depends on the rearing system, location, type of animals, and client education. Owners who are serious about the genetic improvement of their animals have generally detailed records. Breeding and health records are easily obtained for most North American, Australian, and European herds but may be difficult to obtain in other areas of the world.1
Age
The impact of age on reproductive performance is clear, particularly in maiden and older females. In young females, special consideration should be given to the onset of puberty and the ability to carry a pregnancy to term. Breeding young or underdeveloped females usually results in a high incidence of early embryonic death, abortion, complications at birth, and compromised reproductive future of the animal. In addition, early breeding may result in stunted growth. Among domestic species, alpacas and llamas are notorious for a high prevalence of congenital defects. Animals with congenital and possibly hereditary defects should be eliminated from the breeding program to avoid propagation of undesirable traits and undue hardship on females.2–5
Older females should be evaluated carefully for their ability to carry a pregnancy to term without jeopardizing the welfare of the animal. Reproductive performance decreases with increasing age and number of pregnancies. In llamas and alpacas, fertility decreases after age 10 years, mostly because of degenerative changes in the uterus.3
Type of Management
One of the most common causes of reproductive failure is poor management practices, specifically management of breeding. Complete information about management of breeding will allow the clinician to identify potential management errors or problems that may have a negative effect on fertility. These problems are usually associated with herd size and structure; origin of sires (on farm or external); method of breeding (in-hand mating versus free or paddock mating); criteria for mating; pregnancy diagnosis technique; management of birthing; and postpartum, nutritional, and health management of the herd and play an important role in reproductive efficiency. Obese or very thin animals are at risk of decreased reproduction performance because of lack of cyclicity or increased pregnancy loss.
Evaluation of Breeding Records
Evaluation of breeding records should be done at both the herd and individual levels. Evaluation of herd breeding records allows the clinician to have a clear idea about the fertility in the herd and appreciate the overall level of breeding management and reproductive performance. Individual breeding records are important when the animals are valuable. This evaluation should be as complete as possible (Box 17-1). Current breeding records and results of reproductive examination should always be available in an easy-to-read format.
Evaluation of Sexual Behavior
Historical and clinical evaluations of sexual behavior should be obtained, whenever possible. Receptivity to the male is a behavior characterized by the female assuming a sitting sternal position (Kush or cush position). Receptivity to the male is not correlated with follicular dynamics or circulating estrogen levels.5–7 However, rejection of the male (spitting, kicking, or running away) is highly indicative of presence of high level of progesterone, particularly in females that have been receptive prior to a mating.6 This is an inexpensive and relatively efficient method for the verification of ovulation and corpus luteum (CL) formation after mating. The spitting behavior is usually noticed about a week after ovulation and intensifies within the first few weeks of pregnancy.
Examination of the Vulva and Perineum
The examination of the perineal region is usually done on the female restrained in stocks or in a sitting position. In alpacas and llamas, the size of the vulva is relatively small, 2.5 centimeters (cm) and 5 cm in length, respectively. The normal vulva has very thin labia. The perineal body is very small. The vulva lies on a perfect vertical plane 2 to 4 cm below the anus (Figure 17-1). The vulva should be inspected for any discharge, lesions, and abnormal size and conformation. In aged or very thin animals, the labia may lie in a more horizontal plane. Also, the normal vertical conformation of the vulva is lost if scar tissue from previous birthing injuries is present or in the presence of congenital abnormalities (Figure 17-2). In old pluriparous animals, the vulva tends to lose its tone and becomes slightly parted in its ventral aspect.
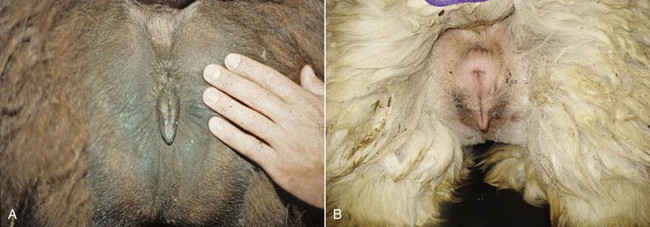
Figure 17-1 Normal Conformation of the Vulva.
A, Llama. B, Alpaca. Note the small perineal body and the prominent clitoris. Tibary A and Anoaussi A. Theriogenology in Camelidae, 2nd Edition, in press, Actes Edition, Institut Agronomique et Vétérinaire Hassan II, Rabat Morocco.
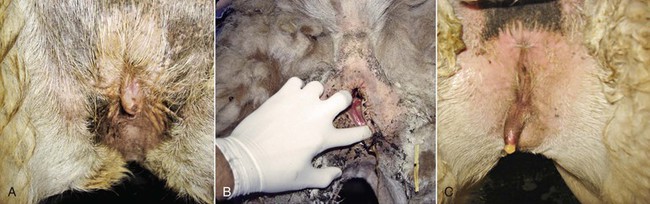
Figure 17-2 Abnormal Conformation of the Vulva.
A, Tilted vulva. B, Third-degree rectovaginal tear. C, Abnormal appearance in an intersexed animal. Tibary A and Anoaussi A. Theriogenology in Camelidae, 2nd Edition, in press, Actes Edition, Institut Agronomique et Vétérinaire Hassan II, Rabat Morocco.
Abnormal size, as in vulvar atresia or stenosis, and position of the vulva in maiden females may occur in cases of congenital problems or in cases of intersexuality (Figure 17-3). In the case of intersexed animals, the vulva presents ambiguous external genitalia with a rudimentary penis. The distance from the anal sphincter to the vulvar (or preputial) opening is usually increased, and the animal shows urinary difficulties.8
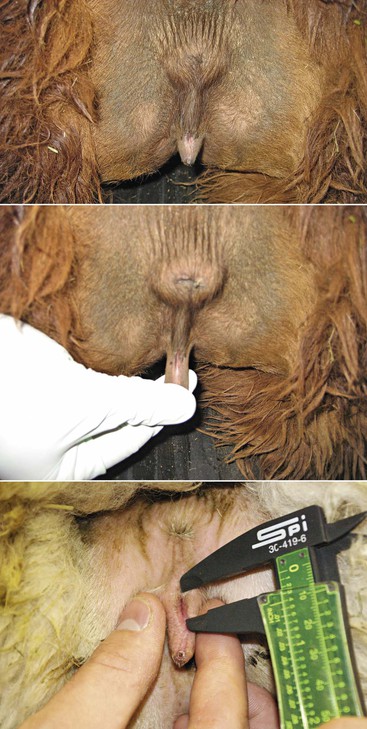
Figure 17-3 Vulvar Atresia.
Small vulvar opening is a common problem in llamas and alpacas. It is important to document the problem by measuring the vulvar opening and verify that no associated vaginal aplasia or persistent hymen exists. Other urinary tract problems have been associated this problem. Tibary A and Anoaussi A. Theriogenology in Camelidae, 2nd Edition, in press, Actes Edition, Institut Agronomique et Vétérinaire Hassan II, Rabat Morocco.
Examination of the external genitalia in the female should be completed with the inspection of the vestibulum and the clitoris. This allows observation of the color of the mucosa, the size of the clitoris, and the presence of discrete discharge. The clitoris in llamas and alpacas is a hard cartilaginous structure that varies in size from one female to another (Figure 17-4).

Figure 17-4 Abnormally long clitoris. Tibary A and Anoaussi A. Theriogenology in Camelidae, 2nd Edition, in press, Actes Edition, Institut Agronomique et Vétérinaire Hassan II, Rabat Morocco.
Examination of the Udder
Examination of the milk is useful for the diagnosis of subclinical mastitis and for isolation of the causative agent in case of acute mastitis. Milk should be examined first using a strip cup (black plate). In cases of acute mastitis, the color may change to a yellowish (presence of pus) or pink (presence of blood) hue and may contain clots or flakes. Watery milk is usually an indication of chronic mastitis.
Transrectal Palpation of the Nonpregnant Female
Transrectal palpation of the internal genitalia offers a great advantage for diagnosis of reproductive disorders, when possible. Per-rectum palpation of the reproductive tract is readily performed in large llamas but is limited by the size of the operator’s hand in small llamas or alpacas. The practitioner should practice as much as possible to develop this skill because it facilitates other examination techniques such as ultrasonography, uterine biopsy, or cervical catheterization for embryo recovery and transfer.1
Clinicians should exercise extreme caution and clearly communicate to clients the risks of transrectal palpation in camelids. Rectal tears or colonic injuries have been reported following excessive manipulation of the rectal wall during palpation or ultrasonography.9 Other problems commonly encountered during per-rectum palpation include bleeding from the anal sphincter or irritation of the rectal mucosa, which can become severe and even lead to partial rectal prolapse. If irritation is severe, we usually start a prophylactic antiobiotherapy. The animal should be placed under observation until the prolapsed tissue is completely retracted to the normal position.
The uterus is often described as being “T” or “Y” shaped, with the horns curling slightly downward and backward at times, and may lie inside of the pelvic cavity or just at the brim of the pelvis in open females. The uterus does not have intercornual ligaments, as observed in ruminants. Its surface is smooth, and the bifurcation is identified as a slight dip or indentation between the horns (Figure 17-5). The parameters recorded during palpation of the uterine horns are size, consistency, tone or contraction, and content. The nonpregnant uterus is always retractable, and the uterine horns are freely movable. The uterus is retracted by gently grasping it in a cupped manner at the level of the bifurcation and then flipping it dorsally. This technique allows palpation of the uterine horns along their entire length as well as evaluation of the broad ligament. Length and diameter of the uterine horns are variable and depend on the age of the animal and number of pregnancies. However, the left horn is invariably larger than the right horn particularly in pluriparous females.
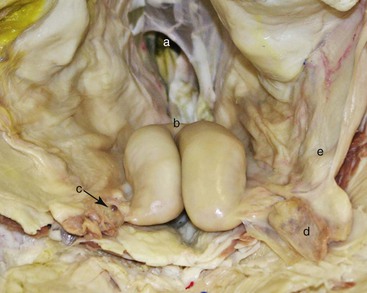
Figure 17-5 In Situ Anatomy of the Reproductive Tract in the Female Alpaca Relevant to Transrectal Palpation and Ultrasonography Technique.
A, Pelvic inlet (rectum removed). B, Bifurcation of the uterine horns. C, Right ovary. D, Left ovarian bursa containing left ovary. E, Left broad ligament. Tibary A and Anoaussi A. Theriogenology in Camelidae, 2nd Edition, in press, Actes Edition, Institut Agronomique et Vétérinaire Hassan II, Rabat Morocco.
Uterine tone and edema increase during the follicular phase and become maximal in the presence of a mature follicle. Uterine tone decreases progressively with regression of the dominant follicle, but the uterus does not become completely relaxed unless a CL is present.1 Relaxation and thinning of the wall of the uterus increase during pregnancy.
Uterine pathologies may be suspected by changes of the shape, size, or limitations in the movement of the uterus. The uterus is extremely flaccid and difficult to discern on palpation in cases of ovarian hypoplasia or dysgenesis (infantile uterus). Segmental aplasia and absence of one uterine horn (uterus unicornis) are relatively common congenital abnormalities in llamas and alpacas.10
Increased size of the uterus in the absence of pregnancy may be attributed to collection of fluid in the uterine cavity (pyometra or mucometra) or incomplete postpartum uterine involution. Accumulation of various amount of fluid may be caused by congenital abnormalities (cervical or vaginal aplasia).1
Once the uterus is retracted, the ovaries can be felt just lateral and caudal to the tip of the horn. The ovaries may become hidden behind the broad ligament because of their long pedicle, in which case it is necessary to free the ovary using a rotational movement on the proper ligament. The ovaries are examined for their presence, size, and general shape by using the thumb and the index finger. Both ovarian follicles and corpora lutea are palpable as in the bovine. The dominant follicle is a smooth, tense, fluid-filled structure protruding from the ovary but without sharp distinction between its wall and the rest of the ovarian surface. The wall of these follicles is thin and may rupture easily if pressure is applied during palpation. The CL can be identified by rectal palpation 3 to 4 days after mating. The CL is softer than the ovarian stroma and protrudes clearly from it (see Chapter 14 Anatomy and Physiology of Reproduction in the Female Llama and Alpaca).
Transrectal Ultrasonography of the Genital Tract
Ultrasonographic examination of the ovarian structure, the uterus, and their relationship to each other at different stages of the cycle has allowed better and more efficient management of breeding.11–13
Examination Technique
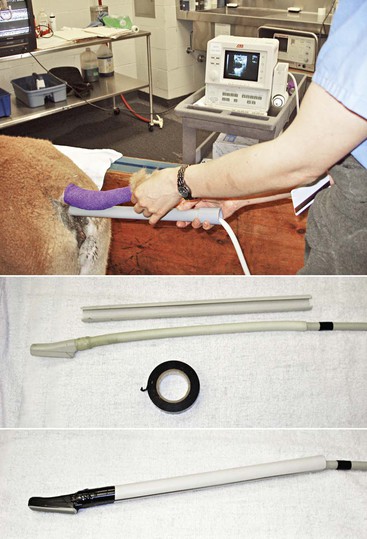
Transrectal ultrasonography of the genital tract is performed with a 7.5-megahertz (MHz) or 5-MHz linear array transducer. In llamas, the examination is preferably performed with the animals in a standing position and restrained in the chute, as described for palpation per rectum when the transducer is manipulated directly per rectum. In alpacas and small llamas, direct manual manipulation of the transducer is not possible. In these cases, the examination is performed with a rigid endorectal (human prostatic) probe or by mounting the linear transducer on a handle (PVC pipe) to allow manipulation without inserting the hand in the rectum (Figure 17-6).13 The tail is wrapped, and the rectal cavity is emptied of fecal material by using two fingers. After evacuation, the rectal cavity is filled with 60 mL of lubricant before proceeding to the ultrasonographic examination of the genital organs. In some situations (when the rectal cavity is very dilated), the use of a coupling gel provides better contact and imaging than use of a regular obstetric lubricant. If animals are transported to a hospital for examination, it is essential to give them some time to urinate and defecate before proceeding with the examination.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree