CHAPTER 3 Clinical Approach to Commonly Encountered Problems
CHANGES IN BODY TEMPERATURE
Assessment of body temperature is an essential part of every physical examination. As with all mammalian species, horses normally maintain their core body temperatures within a narrow range despite extremes in environmental conditions. The core temperatures may vary by approximately 1° C (2° F) between individuals. In adult horses the average normal body temperature is 38.0° C (100.5° F), whereas in neonatal foals the temperature tends to be slightly higher, ranging between 37.8° to 38.9° C (100.0° to 102.0° F). A diurnal variation of up to 1° C (2° F) may occur, with the low point typically in the morning and the peak in the late afternoon.
CONTROL OF BODY TEMPERATURE
The set-point is the crucial temperature that the body attempts to maintain, primarily via neuronal control operating through temperature centers in the hypothalamus.1,2 Peripheral and central thermoreceptors sense changes in ambient and core body temperatures and activate feedback mechanisms that bring the temperature back to the set-point. Specifically, the anterior hypothalamic-preoptic area contains large numbers of heat-sensitive neurons and lower numbers of cold-sensitive neurons that function as temperature detectors. Peripheral receptors, which are generally most sensitive to low temperatures, are located in the skin and in some deep tissues, such as the spinal cord and abdominal viscera, and around certain great veins. The anterior hypothalamic-preoptic area and the peripheral receptors transmit signals into the posterior hypothalamic area, subsequently activating autonomic and behavioral effector responses to regulate body temperature.
When the body temperature is too high, heat loss increases and heat production diminishes. Increasing blood flow to the skin is an effective mechanism for heat transfer from the body core to the surface. In response to changes in core body temperature and environmental temperature, the sympathetic nervous system regulates the degree of vasoconstriction and thus the amount of blood flow. Heat is lost from body surfaces to the surroundings by several physical mechanisms, including radiation, conduction, and convection. Evaporation is also an important mechanism of heat loss in horses.3 The rate of sweating controls to some extent the amount of evaporative heat loss. However, even when the animal is not sweating, water evaporates insensibly from the skin and lungs, causing continual heat loss. In horses evaporative heat loss, primarily through increased sweating but also through increased respiration, becomes more important as the ambient temperature rises and during exercise.3,4 In addition to increased heat loss when the body temperature rises, the horse also decreases temperature further by inhibiting means of heat production, such as shivering, and by behavioral responses, such as seeking shade and wind currents and wading into water.
Mechanisms that increase body temperature come into play when the body temperature is too low.2 Heat is conserved by stimulation of the posterior hypothalamic sympathetic centers, leading to cutaneous vasoconstriction and piloerection. Heat production also increases and may occur through increased muscle activity ranging from inapparent contractions to generalized shivering. Shivering may increase heat production by four to five times baseline. The primary motor center for shivering is in the posterior hypothalamus, which normally is stimulated by cold signals from the peripheral receptors and to some extent the anterior hypothalamic-preoptic area. Signals from heat-sensitive neurons in the anterior-hypothalamic-preoptic area inhibit the center. Digestion of food also contributes to total body heat. Sympathetic stimulation may increase the rate of cellular metabolism, increasing heat production by chemical thermogenesis. Cooling also increases the production of thyrotropin-releasing hormone, ultimately increasing thyroid hormones and cellular metabolism and further contributing to chemical thermogenesis. In addition to these physiologic adaptations, behavioral responses to conserve heat also occur, such as adopting a huddled stance, aggregating in groups, and seeking shelter.
CONDITIONS OF INCREASED BODY TEMPERATURE
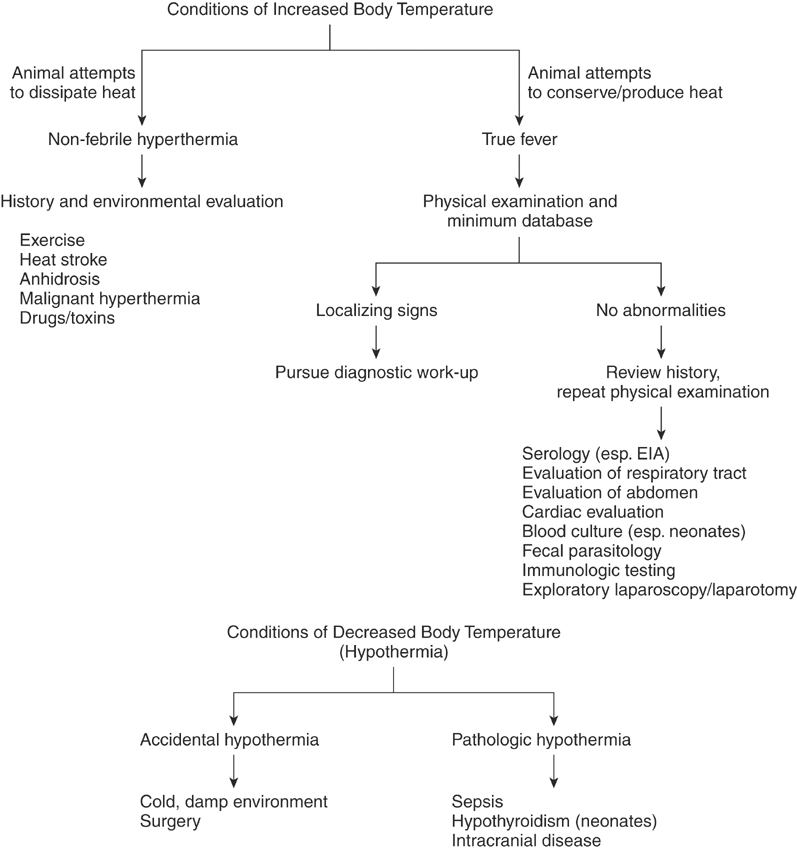
Elevation of the body temperature above normal is one of the most common clinical problems encountered, and although classically associated with infection, a variety of disorders may cause increased body temperature. Veterinarians should distinguish between conditions of hyperthermia, in which the temperature set-point is unaltered, and true fever, in which the set-point actually increases.
Hyperthermia
EXERCISE-RELATED HYPERTHERMIA
During sustained or high-intensity exercise, increased heat production is associated with muscular activity.3,4 The heat produced may exceed the ability of the body to lose heat, resulting in an increased core body temperature. Typically, the temperature returns to normal with rest as heat loss mechanisms remain activated. Elevated temperature also may occur with the intense muscle activity associated with generalized seizures.
HEAT STROKE
Heat stroke occurs when the body temperature rises above a critical temperature, leading to multisystemic problems. In horses signs of heat stroke may develop when the body temperature is above 41.5° C (107° F), which most often occurs in association with exercise in environmentally stressful conditions. Although horses can acclimatize to various weather conditions to some extent, the efficiency of evaporative heat loss may be compromised significantly in hot, humid weather.4,5 Susceptibility to heat stroke may increase if sweating leads to dehydration and electrolyte imbalances. Once the body temperature reaches the critical point, the homeostatic mechanisms of thermoregulation fail, resulting in peripheral vasoconstriction, decreased cardiac output, and decreased blood pressure. Affected horses are lethargic, with weak, flaccid muscles. Prostration, circulatory shock, disseminated intravascular coagulation, multiple organ failure, and death may occur.
ANHIDROSIS
Especially in hot, humid climates, horses may develop anhidrosis, which is characterized by a partial or total loss of the ability to sweat.6 Because of the resulting impaired heat loss, hyperthermia may develop. Clinical signs of poor performance, increased respiratory rate, and poor hair coat also are observable.
MALIGNANT HYPERTHERMIA
Malignant hyperthermia encompasses a group of inherited skeletal muscle disorders in which calcium metabolism is altered.7 Although the condition is most common in human beings and pigs, it has been reported in several species, including horses.8,9 The disorder is characterized by a hypermetabolic state of muscle that generally is induced by halogenated inhalation anesthetics, depolarizing skeletal muscle relaxants, and occasionally local anesthetics or stress. Clinical signs include a rapid increase in core body temperature, skeletal muscle rigidity, and tachycardia. Affected animals may develop significant acidosis and muscle necrosis and in some cases may die. In pigs, malignant hyperthermia has been linked to a single point mutation in the gene for the skeletal muscle ryanodine receptor, but a genetic basis has not yet been established in horses.7
CENTRAL NERVOUS SYSTEM DISORDERS
Any condition affecting those areas of the hypothalamus involved in thermoregulation may alter the body temperature, with hyperthermia being more common than hypothermia.1,2 Thus central hyperthermia occurs in association with a variety of conditions, including hemorrhage, neoplasms or abscesses, infectious/inflammatory changes, and degenerative disorders. Central hyperthermia usually is characterized by a lack of any diurnal variation, absence of sweating, resistance to antipyretic drugs, and excessive response to external cooling.
CERTAIN TOXINS OR DRUGS
Occasionally, hyperthermia has been associated with toxins or drugs. Exposure to compounds that act to uncouple oxidative phosphorylation, such as the wood preservative pentachlorophenol, potentially could cause a significant rise in body temperature.10 Foals treated with the antibiotic erythromycin and possibly other macrolides are at risk of developing hyperthermia.11 Such predisposition has been attributed to a reaction to the erythromycin itself or to an alteration of the thermoregulatory system of the foal by mechanisms not yet described. Environmental conditions may exacerbate the development of hyperthermia, with foals exposed to high ambient temperatures and direct sunlight being at greatest risk.
PATHOGENESIS OF TRUE FEVER
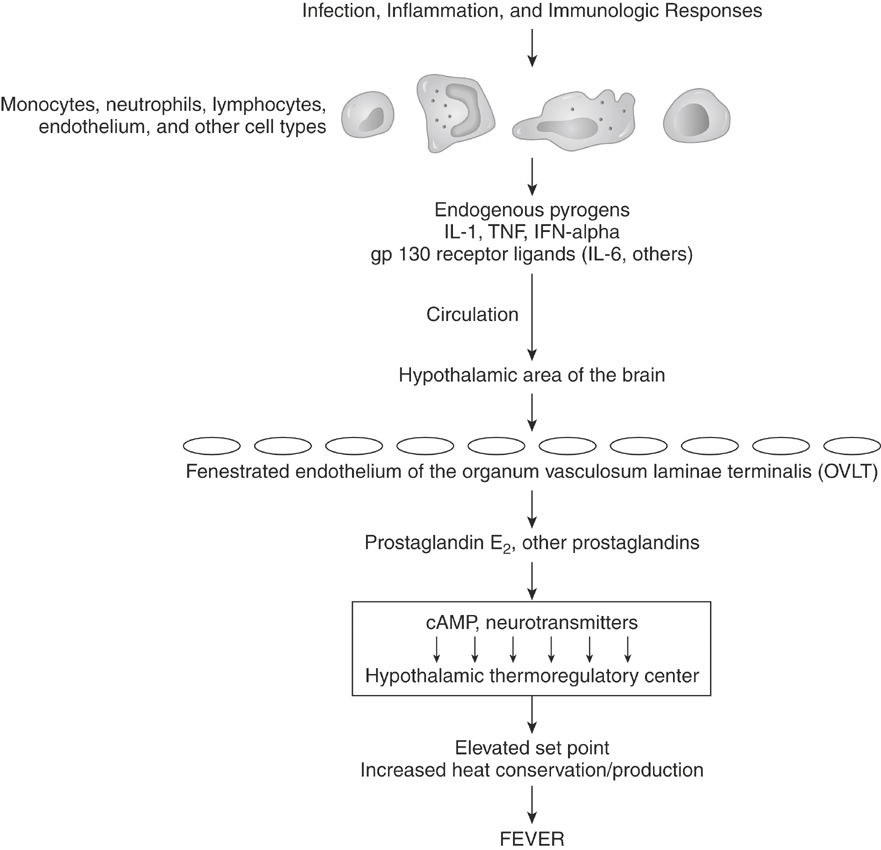
In true fever the set-point for the desired core body temperature increases and then is maintained by the same mechanisms that maintain the normal body temperature. Although primarily associated with infectious diseases, fever is also a prominent component of many inflammatory, immunologic, and neoplastic conditions. Although the pathogenesis of the febrile response is complex, essentially all of these conditions initiate fever by stimulating the release of endogenous pyrogens (Figure 3-1).
Endogenous pyrogens are substances with the biologic property of fever induction.12,13 Initially endogenous pyrogen was assumed to be a single molecule produced by leukocytes—thus the name leukocytic or granulocytic pyrogen. Now, multiple cytokines are known to act as pyrogens, and a variety of cell types produce them, with monocytes and macrophages predominating. Currently, the following cytokines are thought to be intrinsically pyrogenic in that they produce a rapid-onset fever via direct action on the hypothalamus without requiring formation of another cytokine: interleukin-1α (IL-1α) and IL-1β, tumor necrosis factors (TNF) α and β, interferon-α, and IL-6. IL-1α and IL-1β and TNF-α appear to be among the most potent pyrogens. Many endogenous pyrogens use the cell-signaling apparatus gp130. Cytokines that act through this receptor include IL-6, IL-11, oncostatin M, ciliary neurotrophic factor, cardiotropin-1, and leukemic inhibitory factor. From a clinical standpoint, several pyrogenic cytokines are produced during most febrile diseases and contribute to the febrile response.
The precise mechanism of action of pyrogenic cytokines in the central nervous system is still unclear. Endogenous pyrogens probably act on the circumventricular organs or organum vasculosum laminae terminalis (OVLT), a rich vascular network associated with neurons of the preoptic anterior hypothalamus.13–15 Ablation of the OVLT prevents fever after a peripheral injection of endogenous pyrogens but has no effect when endogenous pyrogens are injected directly into the brain tissue.14 In the region of the OVLT the blood-brain barrier is minimal, and endothelial cells lining this region may allow direct movement of endogenous pyrogens into the brain or may release arachidonic acid metabolites in response to endogenous pyrogens, which then move into the brain. The production of arachidonic acid metabolites, particularly prostaglandin E2 via the cyclooxygenase 2 (COX-2) pathway, is clearly important in the pathogenesis of fever because COX inhibitors, and specifically COX-2 inhibitors, effectively reduce the febrile response but have no effect on the normal body temperature. The prostaglandins do not act directly but initiate neuronal signaling by producing a cascade of changes in cyclic nucleotides, calcium, and monoamines leading to a higher set-point in the hypothalamic thermoregulatory center.
Physiologic mechanisms exist to control the febrile response and prevent extremes that are incompatible with life. Multiple feedback mechanisms limit the activity of the pyrogenic cytokines, and many endogenous cryogens or antipyretics have been identified.16,17 For example, IL-10, which can be induced by pyrogenic cytokines, inhibits further production of IL-1 and TNF. Arginine vasopressin and α-melanocyte-stimulating hormone act within the brain to decrease fever.16–19 When administered to human beings, α-melanocyte-stimulating hormone is a much more potent antipyretic than acetaminophen. Nitric oxide also has been shown to have an antipyretic role, mediated by cyclic guanosine monophosphate, in the anterior hypothalamic-preoptic region.20
Pyrogenic cytokines, particularly IL-1 and TNF-α, cause membrane perturbation with an increase in phospholipases and the production of arachidonic acid.12,13 The subsequent production of mediators depends on the metabolic pathways for arachidonic acid in the target tissue. Prostaglandins induced by endogenous pyrogens stimulate the muscle catabolism associated with fever and induce collagenase synthesis from synovial cells, contributing to the muscle and joint pain often seen with fever. Local tissue responses to IL-1β and TNF-α may stimulate afferent neural impulses that lead to behavioral responses associated with fever, such as lethargy and anorexia. As expected, treatment with COX inhibitors can diminish many of the signs of fever.
EFFECTS OF FEVER
Fever is a normal physiologic response with beneficial and adverse effects to the animal. With the exception of some viral infections, the elevation in temperature is generally not high enough to affect pathogens directly. However, studies on bacterial infections in several species have demonstrated an increase in survival with fever, which is thought to be caused primarily by enhanced host defenses.21–23 In addition, the concentration of iron, which is required by many bacteria for multiplication, decreases during the acute phase response.24–26 If the temperature becomes extremely high, many of the beneficial effects are reversed.2,27,28 In rabbits the severity of bacterial infection increases when the body temperature is more than 3° C (5° F) above normal. The increased catabolism, variable anorexia, and increased metabolic rate can lead to muscle wasting and weakness when fever is prolonged. Although seizures induced by fever are uncommon in horses, they can be seen in neonates when the temperature is above 42° C (108° F).29 In debilitated animals prolonged fever has been associated with cardiovascular failure.
APPROACH TO FEVER
Increased body temperature is a common clinical sign with diverse causes (Figure 3-2). Fortunately, in many cases the cause may be readily apparent on the basis of the signalment, history, and physical examination. Conditions of increased temperature, such as exercise-related hyperthermia and malignant hyperthermia, are often apparent from the history. Infectious diseases remain the most common cause of fever, and often localizing clinical signs such as nasal discharge or diarrhea aid in the diagnosis. In other cases an increased temperature may be one component of another obvious condition, such as neoplasm, immune-mediated disease, or a drug reaction.
FEVER OF UNKNOWN ORIGIN
Fever of unknown origin exists when fever is prolonged with no other specific signs. In many cases the cause is a common disease with an unusual presentation. The specific criteria used to define fever of unknown origin in the horse in a review of 63 cases included the following: (1) illness of at least 3 weeks’ duration associated with nonspecific signs, (2) body temperature of at least 38.6° C (101.5° F) on several occasions, and (3) no clear diagnosis after an initial complete blood count and serum biochemical profile.30 The most common cause was found to be infection, which was responsible for 43% of the cases. Other causes included neoplasms in 22% of cases; immune-mediated diseases in 6.5%; and miscellaneous diseases such as toxic hepatopathy, parasitism, and others in 19%. In 9.5% of cases no diagnosis was made. Therefore diagnosis of fever of unknown origin requires a systematic approach with emphasis on the evaluation of infectious disease.
Bacterial endocarditis can cause a fever of unknown origin, although the condition is not as common in horses as in some other species. In the study by Mair, Taylor, and Pinsent, the authors identified endocarditis in 3 of 63 cases of fever of unknown origin.30 In each case a murmur they did not identify initially became apparent several weeks after the onset of illness. Therefore a thorough cardiac evaluation, including echocardiography, is indicated.
Blood cultures are generally most useful in neonates but can yield valuable information in adult horses with fever as well. Ideally, collect three to five samples at least 45 minutes apart when the horse is not on a regimen of antibiotic therapy. Sampling just before and during a temperature rise is most likely to yield a positive culture.
The clinician should consider equine infectious anemia as a differential diagnosis for horses with fever of unknown origin and should perform a serologic examination. Recently, a serologic test for detection of antibodies to the M protein of Streptococcus equi ssp. equi was developed as an aid in the diagnosis of internal abscessation.31 Serologic tests for equine babesiosis, brucellosis, and coccidioidomycosis are also available.
HYPOTHERMIA
Hypothermia occurs when the core body temperature drops below accepted normal values. In clinical cases hypothermia can be characterized as accidental or pathologic (see Figure 3-2). In accidental hypothermia a spontaneous decrease in the core body temperature occurs independent of actual disruption to the thermoregulatory system. These cases often can be identified from the history. Mild accidental hypothermia sometimes occurs with surgical procedures. Most often, accidental hypothermia is associated with exposure to cold or cold, damp environments, which can lead to severe hypothermia and death. Neonates are particularly susceptible to hypothermia, although central thermoregulation through the hypothalamus is normal.29 Sick foals often decrease their activity and nutritional intake and have alterations in circulation. They also have a large ratio of surface area to body weight, enhancing heat loss. Geriatric and otherwise debilitated animals are also at increased risk of hypothermia.
One should consider pathologic causes of hypothermia when no clear reason for accidental hypothermia is evident. Pathologic hypothermia occurs in association with disorders that decrease metabolic activity or directly affect the thermoregulatory center and occurs with endocrine disorders, sepsis, and intracranial disease. In horses hypothyroidism is probably an uncommon clinical problem; however, impaired thermoregulation has been seen in foals with congenital hypothyroidism.32 Lesions of the thyroid gland also have been associated with hypothermia in donkeys.33 Hypothermia has been observed with septicemia and shock, especially in neonates, in which 24% of septic foals were found to have a decreased body temperature.29
DECREASED BODY WEIGHT
Losses in body weight are usually insidious and chronic but may be surprisingly rapid in the face of acute overwhelming systemic infections (Table 3-1). Causes have been classified variously as gastrointestinal, nutritional, infectious, or hypoproteinemic.1,2 Differential mechanisms include decreased feed intake, decreased absorption of nutrients, decreased nutrient utilization, and increased loss of energy or protein leading to a catabolic “sink.”1–3
TABLE 3-1 Mechanisms and Selected Differential Diagnoses for Decreased Body Weight
| Mechanism | Differential Diagnoses |
|---|---|
| Lack of access to appropriate food | |
| Lack of ingestion of available nutrients | |
| Abnormal digestion, absorption, or metabolism of nutrients | |
| Inadequate delivery of nutrients to peripheral tissues | |
| Increased rate of protein and energy use or loss | |
| Primary muscle wasting disorders |
Decreased feed intake may be caused by management factors, poor dentition, dysphagia, or esophageal obstruction. Management factors leading to weight loss may be multifactorial and include inadequate amounts of feed, inadequate quality of feed, or inability of the horse to eat the proper amounts of the feed given. A horse with severe lameness (e.g., chronic laminitis) may not be able to ambulate to the feed source. A horse low on the pecking order in a pasture hierarchy may be unable to eat because it cannot approach the feed without the other horses bullying it and fending it away. The feed must be palatable and digestible. Appropriate amounts and types of concentrates must be fed with consideration to the work schedule or pregnancy status of the horse. Proper investigation of stable feeding practices is described earlier.
Poor dentition may cause the horse not to eat some or all of its grain or hay. Parrot-mouthed horses or aged horses with receding incisor teeth (more than 25 years old) may have difficulty in tearing off grass when grazing. A horse with one or more oral sores from a poorly fitting bit or sharp cheek teeth may exhibit partial or complete inappetence because of pain associated with chewing. Sharp cheek teeth, wave mouth, or step mouth may lead to poor digestion and incomplete absorption of nutrients because of inadequate mastication of hay leading to poor fiber use during the hindgut (cecum) fermentation process.
Dysphagia has many causes, including abnormal prehension, chewing, or swallowing.4 Abnormal prehension can be caused by tongue lacerations; dental, mandibular, or maxillary fractures; damage to nerves supplying the tongue or facial musculature (local trauma, equine protozoal myelitis, or polyneuritis equi); or central neurologic disease (equine protozoal myelitis). Basal ganglia lesions caused by poisoning by ingestion of yellow star thistle or Russian knapweed prevent normal prehension in the pharynx.4 Swallowing abnormalities may be caused by neurologic (equine protozoal myelitis, viral encephalitis, or guttural pouch infection); muscular; or physical obstructions such as strangles, abscesses, or guttural pouch distention.4 Muscular causes include hyperkalemic periodic paralysis in Quarter Horse foals, vitamin E or selenium deficiency in neonates, botulism in neonates and adults, and local trauma subsequent to laryngeal surgery (laryngoplasty).
If the horse with weight loss has been observed to fully ingest adequate amounts of good-quality hay and grain, then decreased feed absorption must be considered the reason for weight loss. Maldigestion and malabsorption are not easily confirmed diagnoses, but tests based on luminal absorption of simple sugars (xylose or glucose tolerance tests) have been used to document malabsorption syndromes.3,5,6 These tests are described in greater detail in Chapter 15. Malabsorption may be caused by parasitism, diarrhea, and inflammatory or neoplastic intestinal disease.
Gastrointestinal parasitism results in weight loss because of several mechanisms.2 Parasites may compete directly for nutrients within the lumen of the bowel. Malabsorption may result from a lack of mucosal integrity, a decrease in intestinal villi size and number (and subsequent decrease in mucosal absorptive surface area), and a decrease in digestive enzymes that originate in the mucosa. Competition of parasites for protein sources may result in decreased availability of amino acids for production of digestive enzymes or mucosal transport proteins. Increased mucosal permeability caused by leakiness in mucosal intercellular bridges may result in mucosal edema and increased transudation of intercellular fluid and its associated electrolytes, amino acids, and sugars into the lumen of the intestine.
Chronic diarrhea may result in partial or complete anorexia, which contributes directly to weight loss. More rapid (decreased) gastrointestinal transit time results in increased losses of incompletely digested dietary feedstuffs. Malabsorption may result from decreased transit time and from villus blunting caused by specific pathogens, such as in viral diarrhea (see Chapter 15). Bacterial pathogens may compete directly for luminal nutrients. Mucosal invasion by viral and bacterial pathogens may cause mild to severe degrees of mucosal sloughing (ulcers), which results in maldigestion, malabsorption, and increased mucosal losses of intercellular fluid (e.g., in parasitism).
Assuming that the horse has adequate feed intake and absorption, inappropriate hepatic use of amino acids and sugars must be considered as a differential diagnosis for weight loss. Chronic liver disease may result in weight loss because of inappetence, maldigestion (caused by inadequate bile acid production), and inadequate or improper processing of amino acids into normal plasma proteins in the liver. These abnormalities may result in lowered concentrations of serum albumin, liver-dependent clotting factors (factors II, VII, IX, and X), and total plasma or serum protein. Lowered circulating proteins (especially albumin) may result in decreased plasma colloid osmotic pressure and thus may manifest as peripheral dependent edema in the distal limbs, pectoral region, and ventral midline. This peripheral edema may mask further weight loss by making the torso of the horse appear to be heavier than it actually is. Decreases in clotting factors may result in bleeding diatheses. Hyperlipemia, hyperlipidemia, fatty liver syndrome, and ketosis may be seen in poorly fed ponies and in miniature horses with acute anorexia or overwhelming energy demands, such as pregnancy or lactation.7
Increased loss of protein or energy is a common cause of decreased body weight in horses. Luminal losses of fluid, electrolytes, and nutrients were described earlier for intestinal parasitism and diarrhea. Acute inflammatory protein losses may occur into major body cavities in overwhelming infections such as pleuritis or peritonitis. Chronic abscessing pneumonia, pleuritis, and peritonitis often result in increased, rather than acutely decreased, serum total protein because of increased γ-globulin production in response to chronic antigenic stimulation from the chronic infection. These chronic infections also usually have weight loss as an additional clinical sign because of the continuing catabolic processes associated with the infection itself. Equine infectious anemia is a type of persistent systemic infection that in its symptomatic form may result in chronic weight loss and varying levels of anemia.8 Asymptomatic equine infectious anemia carriers may have no weight loss or other obvious clinical signs but can infect pasture mates via vector transmission.
Protein-losing enteropathy is not a definitive diagnosis but rather is a group of diseases, each of which results in luminal losses of fluid, electrolytes, plasma proteins, and nutrients. Mechanisms of protein and fluid loss were described earlier for intestinal parasitism and diarrhea. Gastrointestinal ulcers, especially in the right dorsal colon, have been reported to result in lowered serum total protein and weight loss.9 One of the early indications of nonsteroidal anti-inflammatory drug toxicity is detection of a lowered serum total protein. Horses with such a condition also may manifest varying degrees of inappetence and colic, especially during the immediate postprandial period. Intestinal neoplasms (usually lymphosarcoma) often manifest as a protein-losing enteropathy with weight loss.10
Acute or chronic renal diseases, especially involving glomerulonephritis, can result in urinary protein loss and subsequent body weight loss.2 Horses with this condition may have polyuria and polydipsia as associated clinical signs. Owners or handlers often report polyuria as increased wetness in stall bedding. The veterinarian should question owners thoroughly regarding the water intake of the horse. The veterinarian may need to observe stable watering habits, often including actually measuring the volume of the water buckets to establish definitively the presence of polydipsia. Turning off automatic water feeders in the stall or pasture and offering the horse measured volumes of water from additional buckets may be necessary to establish a diagnosis of polydipsia. Urine puddles in stalls or collected urine samples may foam excessively because of increased protein concentrations. Increased urinary protein concentrations can be diagnosed quickly on the farm with the proper interpretation of urine dipstick protein indicators.
Neoplasms or abscesses within the thorax or abdomen serve as catabolic energy and protein sinks, resulting in chronic weight loss.2,10,11 Chronic pain, such as that associated with severe, unresponsive laminitis, results in increased catabolism and weight loss, probably because of chronically elevated systemic catecholamine levels. Increased circulating epinephrine and norepinephrine levels result in a whole-body catabolic state with increased breakdown of stored energy sources and ultimately result in chronic weight loss. Similar weight loss caused by systemic catabolism can result from chronically elevated serum cortisol associated with pituitary adenoma and secondary hyperadrenocorticism.
Approach to the Diagnosis of Weight Loss
Peritoneal fluid analysis documents the presence of a transudate (equivocal infection) or exudate (probable infection).12,13 Aerobic and anaerobic peritoneal fluid cultures should be performed if intra-abdominal infection is suspected. Exfoliative cytologic examination rarely may document the presence of neoplastic cells from intra-abdominal neoplasms.2,10–14
Nonroutine tests should be performed only as indicated and should include oral absorption tests (see Chapter 15) and biopsies of the liver, kidney, or intestinal wall. Abdominal or thoracic ultrasonography should help in ruling out abnormalities of the liver or kidneys and may document the presence of abnormal fluid (peritonitis or pleuritis) or masses (abscesses or neoplasms). Cardiac ultrasound should be definitive in the event of a murmur and suspected heart failure. Radiography also may be helpful to document the presence of thoracic masses or chronic obstructive pulmonary disease, but increased pleural fluid obscures visualization of other intrathoracic structures.
INCREASED BODY WEIGHT
Ponies seem to be particularly susceptible to obesity, perhaps because their size renders them more easily overfed. However, at least one author has proposed that this tendency toward obesity in ponies receiving modern confinement diets may be because of their having evolved in the inhospitable ice age climates of northern Europe.15 In that era the lack of readily available grazing feedstuffs might have placed greater selection pressure on survival of ponies with more efficient dentition and better nutrient and fluid absorption from the gastrointestinal tract. The author argues that those ponies that had greater feed conversion efficiency would have been stronger, had longer lives, and been more available for breeding. Current illustrations of this theory may lie in the Welsh and Connemara pony breeds that still thrive and flourish in the wild in the inhospitable north Atlantic climates of the western coasts of Wales and Ireland, respectively.
Obesity has also been associated with equine metabolic syndrome, also known as peripheral Cushing’s disease or prelaminitic metabolic syndrome.16–18 Affected horses are insulin resistant and at increased risk of developing laminitis. They may exhibit either generalized obesity or regional adiposity, typically manifested as a cresty neck or fat pads next to the tailhead. Although equine metabolic syndrome has been recognized in many breeds, some breeds, including ponies, Morgans, Paso Finos, and Norwegian Fjords, appear to be predisposed. Certain management practices, such as the provision of a starch-rich, high glycemic–index diet to horses that are relatively inactive, may contribute to the development of the syndrome. Previously, affected horses were sometimes assumed to suffer from hypothyroidism, but recent studies do not support a primary role for thyroid dysfunction in equine metabolic syndrome.
Horses with pituitary pars intermedia dysfunction (PPID; equine Cushing’s syndrome) may also have insulin resistance and abnormal accumulation of fat.19 Although horses with PPID often have loss of muscle mass or weight loss, some individuals will also have excess fat deposition, especially along the crest of the neck, over the tailhead, in the sheath or in the supraorbital fossa. The presence of additional clinical signs, such as hirsutism, as well as specific testing for PPID, will help to differentiate these horses from those with insulin resistance and fat accumulation resulting from metabolic syndrome.
Hypothyroidism has been reported to be associated with weight gain in horses and obesity and infertility in broodmares.15,20 However, evidence for hypothyroid-associated weight gain and infertility was lacking in surgically created hypothyroid pony21 and Quarter Horse22 subjects. Also, a clinical study demonstrated that decreased thyroid function was uncommon in broodmares and was not a common cause of infertility.23 In general, hypothyroidism remains a somewhat controversial disorder in adult horses, both because of difficulties in accurately diagnosing the condition and the numerous extrathyroidal factors that can affect thyroid function.24–27 Because resting thyroid hormone concentrations can vary widely depending on a number of factors, documentation of hypothyroidism may require performance of a thyroid-stimulating hormone or thyroid-releasing hormone stimulation test or determination of thyroid-stimulating hormone concentrations.25–27 The diagnosis and treatment of thyroid dysfunction is described in greater detail elsewhere in this text.
Approach to the Diagnosis of Increased Body Weight
Major differential diagnoses for increased body weight include overfeeding; pregnancy; metabolic syndrome; and other conditions that result in abdominal distention, such as bloat, ascites, uroperitoneum, fetal hydrops, and rupture of the prepubic tendon or abdominal wall musculature. The latter conditions are described in greater detail in Chapter 18.
Feeding practices should be investigated and observed firsthand if necessary. A positive pregnancy status should be an easy historical and rectal diagnosis. Most hematologic and biochemical tests are normal in the pregnant or simply overweight horse. Horses should be evaluated for insulin resistance and PPID. Thyroid status should be assessed appropriately, not by simple resting thyroid hormone concentrations.24–26
APPROACH TO POOR PERFORMANCE
Determining the cause of poor performance in those horses without overt clinical disease often is challenging.1–4 In a study by Martin, Reef, Parente, et al. of 348 cases of poor performance, a definitive diagnosis was established in 73.5% of cases after in-depth examination, which included the use of a high-speed treadmill.3 Subtle abnormalities may be sufficient to impair performance, and in some cases problems may be evident only during exercise, contributing to the difficulty of making a diagnosis. Additionally, multiple problems may occur concurrently. In a study by Morris and Seeherman of 275 racehorses with a history of poor racing performance, 84% were found to have more than one abnormality.2 Therefore determining the actual clinical significance of any given problem may be difficult.
Equine athletes presented for poor performance should undergo a comprehensive evaluation, the basic components of which include a history, detailed physical examination, and laboratory screening. The clinician should emphasize examination of the respiratory, musculoskeletal, and cardiovascular systems because these systems most often are linked to performance problems. In many cases standardized exercise testing, generally on a high-speed treadmill, is critical in identifying the problem. Endoscopic examination of the upper airways during exercise has proved particularly useful.