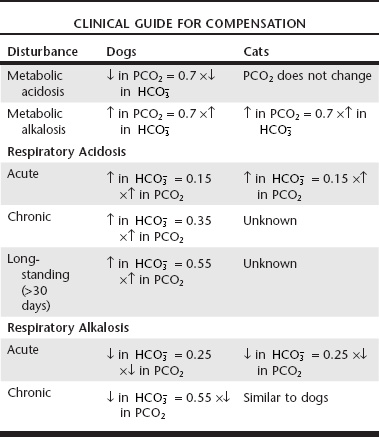
Web Chapter 1 Any alteration in acid-base equilibrium sets into motion a compensatory response by either the lungs or the kidneys. The compensatory response attempts to return the ratio between PCO2 and WEB TABLE 1-1 Compensatory Response in Simple Acid-Base Disturbances in Dogs and Cats Modified from de Morais HSA, DiBartola SP: Ventilatory and metabolic compensation in dogs with acid-base disorders, J Vet Emerg Crit Care 1(2):39, 1991; and de Morais HSA, Leisewitz A: Mixed acid-base disorders. In DiBartola SP, editor: Fluid, electrolyte, and acid-base disorders, ed 3, Philadelphia, 2006, Elsevier, p 296. Calculating the various gaps and gradients can be useful in evaluation of acid-base disorders (Web Box 1-2). Increases in the anion gap (AG) and strong ion gap (SIG) are associated with increases in concentration of unmeasured anions, both strong (e.g., lactate, acetoacetate, β-hydroxybutyrate, strong anions of renal failure) and weak (e.g., phosphate). The AG also is used to differentiate between hyperchloremic (normal AG) and high-AG metabolic acidoses. The AG in normal dogs and cats is mostly a result of the net negative charge of proteins and thus is heavily influenced by protein concentration, especially albumin. At plasma pH of 7.4 in dogs, each decrease of 1 g/dl in albumin concentration is associated with a decrease of 4.1 mEq/L in the AG (Constable and Stämpfli, 2005). The SIG is not affected by changes in albumin concentration, and an increase in unmeasured strong anions is suspected whenever SIG is less than −5 mEq/L. The SIG has not been clinically tested in dogs and cats, but its derivation is sound, and it is superior to the AG for detecting increases in unmeasured strong anions in other species. Frequently, patients with respiratory acidosis or alkalosis also are hypoxemic. When determining management options, it is important to differentiate between hypoxia from primary lung disease (e.g., ventilation-perfusion mismatching) and alveolar hypoventilation by calculating the alveolar-arterial oxygen gradient, or (A – a) O2 gradient. Values less than 15 mm Hg generally are considered normal. If the (A − a) O2 gradient is increased, a component of the hypoxemia results from ventilation-perfusion mismatching, although it may be increased in some patients with extrapulmonary disorders. Clinically, a normal gradient excludes pulmonary disease and suggests some form of central alveolar hypoventilation or an abnormality of the chest wall or inspiratory muscles. To increase the specificity of the test to diagnose ventilation-perfusion mismatch, only patients with (A − a) O2 gradient values of more than 25 mm Hg should be considered abnormal (Johnson and de Morais, 2012). These patients are likely to have primary pulmonary disease, but extrapulmonary disorders cannot be completely ruled out. Respiratory alkalosis, or primary hypocapnia, is characterized by decreased PCO2, increased pH, and a compensatory decrease in
Acid-Base Disorders
Stepwise Approach
Calculate the Expected Compensation
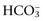 to normal and thereby minimize the pH change. A primary increase or decrease in one component is associated with a predictable compensatory change in the same direction in the other component (Web Table 1-1). Adaptive changes in plasma
to normal and thereby minimize the pH change. A primary increase or decrease in one component is associated with a predictable compensatory change in the same direction in the other component (Web Table 1-1). Adaptive changes in plasma 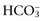 in respiratory disorders occur in two phases: acute and chronic. In respiratory acidosis, the first phase represents titration of nonbicarbonate buffers, whereas in respiratory alkalosis, the first phase represents release of H+ from nonbicarbonate buffers within cells. This response is completed within 15 minutes. The second phase reflects renal adaptation and consists of increased net acid excretion and increased
in respiratory disorders occur in two phases: acute and chronic. In respiratory acidosis, the first phase represents titration of nonbicarbonate buffers, whereas in respiratory alkalosis, the first phase represents release of H+ from nonbicarbonate buffers within cells. This response is completed within 15 minutes. The second phase reflects renal adaptation and consists of increased net acid excretion and increased 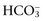 reabsorption (decreased Cl− reabsorption) in respiratory acidosis and decreased net acid excretion in respiratory alkalosis. Adaptive respiratory response to metabolic disorders begins immediately and is complete within hours. Some guidelines for use of compensatory rules from Web Table 1-1 are presented in Web Box 1-1.
reabsorption (decreased Cl− reabsorption) in respiratory acidosis and decreased net acid excretion in respiratory alkalosis. Adaptive respiratory response to metabolic disorders begins immediately and is complete within hours. Some guidelines for use of compensatory rules from Web Table 1-1 are presented in Web Box 1-1.
Calculate Gaps and Gradients
Strong Ion Gap and Anion Gap
Alveolar-Arterial Oxygen Gradient
Respiratory Acid-Base Disorders
Disorders of PCO2
Respiratory Alkalosis
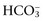 concentration in the blood. Respiratory alkalosis occurs whenever the magnitude of alveolar ventilation exceeds that required to eliminate the CO2 produced by metabolic processes in the tissues. Common causes of respiratory alkalosis include stimulation of peripheral chemoreceptors by hypoxemia, primary pulmonary disease, direct activation of the brainstem respiratory centers, overzealous mechanical ventilation, and situations that cause pain, anxiety, or fear (Web Box 1-3). It is difficult to attribute specific clinical signs to respiratory alkalosis in the dog and cat. The clinical signs usually are caused by the underlying disease process and not by the respiratory alkalosis itself. However, in humans, headache, light-headedness, confusion, paresthesias of the extremities, tightness of the chest, and numbness around the mouth have been reported in acute respiratory alkalosis. If the pH exceeds 7.6 in respiratory alkalosis, neurologic, cardiopulmonary, and metabolic consequences may arise. Such a pH only can be achieved in acute respiratory alkalosis before renal compensation ensues. Alkalemia results in arteriolar vasoconstriction that can decrease cerebral and myocardial perfusion. In addition, hyperventilation (PCO2 < 25 mm Hg) causes decreased cerebral blood flow, potentially resulting in clinical signs such as confusion and seizures. Treatment of respiratory alkalosis should be directed at relieving the underlying cause of the hypocapnia; no other treatment is effective. Respiratory alkalosis severe enough to cause clinical consequences for the animal is uncommon. Hypocapnia itself is not a major threat to the well-being of the patient. Thus the underlying disease responsible for hypocapnia should receive primary therapeutic attention.
concentration in the blood. Respiratory alkalosis occurs whenever the magnitude of alveolar ventilation exceeds that required to eliminate the CO2 produced by metabolic processes in the tissues. Common causes of respiratory alkalosis include stimulation of peripheral chemoreceptors by hypoxemia, primary pulmonary disease, direct activation of the brainstem respiratory centers, overzealous mechanical ventilation, and situations that cause pain, anxiety, or fear (Web Box 1-3). It is difficult to attribute specific clinical signs to respiratory alkalosis in the dog and cat. The clinical signs usually are caused by the underlying disease process and not by the respiratory alkalosis itself. However, in humans, headache, light-headedness, confusion, paresthesias of the extremities, tightness of the chest, and numbness around the mouth have been reported in acute respiratory alkalosis. If the pH exceeds 7.6 in respiratory alkalosis, neurologic, cardiopulmonary, and metabolic consequences may arise. Such a pH only can be achieved in acute respiratory alkalosis before renal compensation ensues. Alkalemia results in arteriolar vasoconstriction that can decrease cerebral and myocardial perfusion. In addition, hyperventilation (PCO2 < 25 mm Hg) causes decreased cerebral blood flow, potentially resulting in clinical signs such as confusion and seizures. Treatment of respiratory alkalosis should be directed at relieving the underlying cause of the hypocapnia; no other treatment is effective. Respiratory alkalosis severe enough to cause clinical consequences for the animal is uncommon. Hypocapnia itself is not a major threat to the well-being of the patient. Thus the underlying disease responsible for hypocapnia should receive primary therapeutic attention.

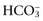 concentration or base excess (BE). The SID is the difference between all strong cations and all strong anions. Strong ions are fully dissociated at physiologic pH and therefore exert no buffering effect. However, strong ions do exert an electrical effect because the sum of completely dissociated cations does not equal the sum of completely dissociated anions. Because strong ions do not participate in chemical reactions in plasma at physiologic pH, they act as a collective positive unit of charge, the SID. The quantitatively most important strong ions in plasma are Na+, K+, Ca2+, Mg2+, Cl−, lactate, β-hydroxybutyrate, acetoacetate, and
concentration or base excess (BE). The SID is the difference between all strong cations and all strong anions. Strong ions are fully dissociated at physiologic pH and therefore exert no buffering effect. However, strong ions do exert an electrical effect because the sum of completely dissociated cations does not equal the sum of completely dissociated anions. Because strong ions do not participate in chemical reactions in plasma at physiologic pH, they act as a collective positive unit of charge, the SID. The quantitatively most important strong ions in plasma are Na+, K+, Ca2+, Mg2+, Cl−, lactate, β-hydroxybutyrate, acetoacetate, and 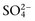 . The influence of strong ions on pH and
. The influence of strong ions on pH and 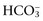 concentration can always be summarized in terms of the SID. Changes in SID of a magnitude capable of altering acid-base balance usually occur as a result of increasing concentrations of Na+, Cl−,
concentration can always be summarized in terms of the SID. Changes in SID of a magnitude capable of altering acid-base balance usually occur as a result of increasing concentrations of Na+, Cl−, 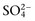 , or organic anions or decreasing concentrations of Na+ or Cl−. An increase in SID (by decreasing Cl− or increasing Na+) causes a strong ion (metabolic) alkalosis, whereas a decrease in SID (by decreasing Na+ or increasing Cl−,
, or organic anions or decreasing concentrations of Na+ or Cl−. An increase in SID (by decreasing Cl− or increasing Na+) causes a strong ion (metabolic) alkalosis, whereas a decrease in SID (by decreasing Na+ or increasing Cl−, 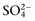 , or organic anions) causes a strong ion (metabolic) acidosis. The main nonvolatile plasma buffers that constitute Atot act as weak acids at physiologic pH (e.g., phosphate, imidazole [histidine] groups on plasma proteins). An increase in the total concentration of phosphate leads to Atot (metabolic) acidosis, whereas a decrease in albumin concentration causes Atot (metabolic) alkalosis.
, or organic anions) causes a strong ion (metabolic) acidosis. The main nonvolatile plasma buffers that constitute Atot act as weak acids at physiologic pH (e.g., phosphate, imidazole [histidine] groups on plasma proteins). An increase in the total concentration of phosphate leads to Atot (metabolic) acidosis, whereas a decrease in albumin concentration causes Atot (metabolic) alkalosis.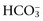 is changing in the same direction that pH changed. There are four classic primary acid-base disorders: respiratory alkalosis, respiratory acidosis, metabolic alkalosis, and metabolic acidosis. Metabolic acid-base disorders can be further divided based on changes in SID or Atot.
is changing in the same direction that pH changed. There are four classic primary acid-base disorders: respiratory alkalosis, respiratory acidosis, metabolic alkalosis, and metabolic acidosis. Metabolic acid-base disorders can be further divided based on changes in SID or Atot.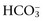 and the compensatory mechanisms affecting these measurements. Lack of appropriate compensation is evidence of a mixed acid-base disorder. Compensation is said to be inappropriate if a patient’s PCO2 differs from expected PCO2 by more than 2 mm Hg in a primary metabolic process or if a patient’s
and the compensatory mechanisms affecting these measurements. Lack of appropriate compensation is evidence of a mixed acid-base disorder. Compensation is said to be inappropriate if a patient’s PCO2 differs from expected PCO2 by more than 2 mm Hg in a primary metabolic process or if a patient’s 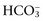 differs from the expected
differs from the expected 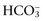 by more than 2 mEq/L in a respiratory acid-base disorder.
by more than 2 mEq/L in a respiratory acid-base disorder.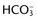 + Cl–)
+ Cl–)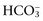 have a tendency to change in opposite directions in hypochloremic alkalosis and hyperchloremic acidosis. The contribution of Cl− to changes in BE and
have a tendency to change in opposite directions in hypochloremic alkalosis and hyperchloremic acidosis. The contribution of Cl− to changes in BE and 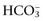 can be estimated by calculating the chloride gap (see Web Box 2-2). Chloride gap values greater than 4 mEq/L are associated with hypochloremic alkalosis, whereas values less than −4 mEq/L are associated with hyperchloremic acidosis. Whenever sodium concentration is normal, the difference between the sodium and chloride concentrations ([Na+] − [Cl−]) can be used. Normally, [Na+] − [Cl−] is approximately 36 mEq/L in dogs and cats. Values greater than 40 mEq/L are an indication of hypochloremic alkalosis, whereas values less than 32 mEq/L are associated with hyperchloremic acidosis.
can be estimated by calculating the chloride gap (see Web Box 2-2). Chloride gap values greater than 4 mEq/L are associated with hypochloremic alkalosis, whereas values less than −4 mEq/L are associated with hyperchloremic acidosis. Whenever sodium concentration is normal, the difference between the sodium and chloride concentrations ([Na+] − [Cl−]) can be used. Normally, [Na+] − [Cl−] is approximately 36 mEq/L in dogs and cats. Values greater than 40 mEq/L are an indication of hypochloremic alkalosis, whereas values less than 32 mEq/L are associated with hyperchloremic acidosis.

