CHAPTER 134 Cervid Semen Collection and Freezing
SEASONALITY
The cervid species mentioned in this section (wapiti, red deer, whitetailed deer, fallow deer, and sika deer) are all seasonal breeders. Because of the relationship between changing daylight length and fertility, there is a limited window of opportunity to collect viable semen1,2 (Fig. 134-1).
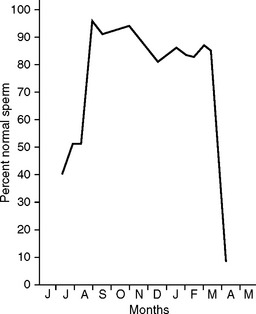
Fig. 134-1 Spermiogram of adult wapiti bulls in the northern hemisphere.
(Adapted from Haigh JC, Barth AD, Cates WF, et al: Electroejaculation and semen evaluation of wapiti. In Fennessy PF, Drew KR: Biology of deer production. Wellington: The Royal Society of New Zealand, 1985, pp 197–203.)
This window can be manipulated early via artificial lights or the use of melatonin implants. Melatonin implants have been successfully used to advance the breeding season of red deer.1 Starting 3 months before the normal breeding season, using one implant of Regulin (Regulin, Schering Agrochemical, Ltd., NSW, Australia) per month, it was possible to obtain good quality semen 1 month before other untreated bulls of the same age.2
However, there were undesirable side effects. These bulls rutted early when the temperatures were hot, and then changed into a summer coat in late winter when the temperatures were cold. They also showed irregular antler growth, going through two antler cycles in 1 year.2
At the beginning of the breeding season, a higher percentage of secondary abnormalities can be seen in the semen sample. The timing of this varies between individual males. Similarly, individual males will display a higher percentage of secondary abnormalities toward the end of the breeding season. In some instances an excess of 50% of sperm in one ejaculate will show distal droplets before cessation of viable sperm production.1
METHOD OF COLLECTION
Under Anesthetic
Initially most cervid species were collected this way.1 However, from the late 1980s onward the method of choice to collect red deer and wapiti semen involved the use of no drugs, and a squeeze or chute.1
If wapiti or red deer are in full antler it may still be necessary to tranquilize or anesthetize them. Drugs used to induce lateral recumbency are listed in Table 134-1. They are either narcotic-based mixtures or a mixture of xylazine HCl and Telazol.1,3
Fentazine or the carfentanyl/xylazine mix is reversed with tolazoline hydrochloride (Tolazine, Lloyd Laboratories, Shenandoah, Iowa) (2–4 mg/kg, given half IM, half IV) or yohimbine hydrochloride (Antagonil, Vetrepharm, London, Ontario, Canada) (1.0–1.25 mg/kg) to reverse the xylazine, as well as Narcan (Naloxone hydrochloride injection, DuPont Pharmaceuticals, Dorval, Quebec City, Canada) to reverse the narcotic (Table 134-2). The more nervous smaller cervid species are best done under anesthesia. The drug combination of choice for whitetailed deer bucks is xylazine/Telazol mix. This is reversed with yohimbine or tolazoline hydrochloride (doses as previously given).
Cervids collected under anesthetic are in lateral recumbency with an assistant holding the back legs and another assistant holding the head.1
EQUIPMENT
The group uses a Lane Pulsator III electroejaculator (Lane Pulsator III, Lane Manufacturing Inc., Aurora, CO) on all species. A sheep probe is ideal for the smaller species, and hand-crafted probes are used for wapiti and red deer (size 4 cm diameter for red deer and 6 cm diameter for wapiti). These can have two or three ventrally placed electrodes.4
Collection vessels used are normally specially blown bell-shaped glasses with a protective water jacket kept at 36° C. Thick glass-bottom shot glasses can be used in warmer climates. Standard bovine rubber collection sheaths and test tube combinations can also be used, but these are more prone to extra contamination from the prepuce and abdomen. The collection vessels can be kept in a water bath or an incubator and returned quickly to either, as the fraction is collected.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




