CHAPTER 2 Cardiovascular Infections
All components of the cardiovascular system, from cardiac tissues to blood vessels, are susceptible to infection. Fortunately, these conditions are relatively uncommon in the horse, but they can be devastating when they occur. Viral or bacterial infection can also act as a trigger for immune-mediated disorders, such as pericarditis and myocarditis. Fever is a common clinical feature of cardiovascular infections, and specific localizing signs will vary depending on the specific site of infection. In general, successful treatment relies on appropriate antimicrobial therapy. In many cases, however, systemic inflammatory response syndrome is a prominent feature, and supportive therapy is important and challenging in these severely compromised individuals.
INFECTIVE ENDOCARDITIS
Etiology and Pathogenesis
Infective endocarditis (IE) is an uncommon but frequently fatal disorder in horses. Endocardial lesions have been reported in association with Lyme borreliosis1 and infection with Shigella equirulis.2 However, review of clinical and pathologic reports of equine IE published since 1980 (34 cases), 3–18,29,31,32 together with an additional six cases seen at the author’s clinic, has demonstrated that a range of microorganisms may be implicated in equine IE. Actinobacillus equuli,3–5 Pasteurella caballi,6 Pasteurella/Actinobacillus spp.,7,8 Pseudomonas spp.,9,10 Escherichia coli,7,11 Corynebacterium spp.,7 Bacillus spp.,7 Serratia marcescens,12 Erysipelothrix rhusiopathiae,13 coagulase-positive Staphylococcus spp.,14 α-hemolytic7 and β-hemolytic15 Streptococcus spp., and Streptococcus equi subsp. equi16 are reported as causes of IE in horses, with no one organism emerging as distinctly more prevalent than the others. Pasteurella/Actinobacillus spp. (6 of 32 reported cases, 18.8%; 95% confidence intervals [CI] 5.2%-32.3%) and Pseudomonas spp. (3 of 32 cases, 9.4%; CI 0%-19.5%) occur more than once in this literature series, whereas the other organisms were each identified in one case only.
Rhodococcus equi was isolated from synovial and bony material removed surgically from a foal with septic osteoarthritis and mural IE, but blood culture from that foal yielded Escherichia coli.11 A blood culture from a 14-year-old mixed-breed gelding with aortic IE in the author’s clinic also yielded R. equi. That horse had no apparent immunosuppression or other reason to have become infected with R. equi and recovered after 6 weeks of treatment with trimethoprim-sulfonamide and rifampin. Although not a typical skin commensal, R. equi may have been a contaminant introduced during the blood collection. Fungal IE has been attributed to Aspergillus species in a horse with disseminated aspergillosis affecting the lungs, intestine, and peritoneal cavity, as well as the mitral valve and left ventricular wall.17 In another report, Candida species affected the aortic valve and right atrial wall in an 11-year-old Thoroughbred.9 In many cases of IE the causative microorganisms cannot be determined. Neither blood nor postmortem cultures allowed identification of a causative organism in 7 of 32 horses (22%; CI 7.6%-36.2%) in which culture was attempted.7,9,18,19
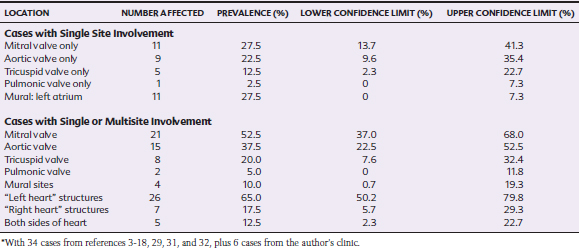
A combination of endothelial damage and bacteremia are prerequisites for the development of IE.20,21 Preexisting heart disease is present in 42% to 98% of human IE patients, and 4% to 13% have congenital defects such as ventricular septal defect (VSD), with preexisting valvular regurgitation in most of the remaining patients.22 Endothelial damage caused by the effects of high-velocity jets and turbulence leads to deposition of complexes of platelets and fibrin, which in turn are susceptible to colonization by bacteria or fungi during bacteremia or fungemia.20,21 In horses the structures on the left side of the heart are most likely to be affected by IE, with the mitral valve affected slightly more often than the aortic valve, despite that preexisting valvular lesions are most likely to be present on the aortic valve23 (Table 2-1). Mural endocarditis occurs less often in horses, possibly because an association between IE and VSD, which is an important predisposing factor in human IE, has not been identified in the horse, although this is a fairly common congenital abnormality in certain breeds, such as the Standardbred, Arabian,24 and Welsh Mountain pony.25
The portal of entry of the causative microorganism is often not apparent. In humans, potential routes include dental infection and procedures, surgery, endoscopy, intravenous (IV) catheters, drug abuse, and infection of the skin, lungs, bowel, and urinary tract.22 No established association exists between bacteremia and dental procedures in horses, but IE has occurred after repulsion of the first molar by trephination of the maxillary sinus in a case of endodontic infection caused by Fusobacterium necrophorum.26 A 13-year-old mixed-breed mare at the author’s clinic had concurrent temporohyoid osteoarthropathy and guttural pouch empyema from which β-hemolytic Streptococcus spp. were isolated, although blood culture was negative. Septic jugular thrombophlebitis is considered a risk factor for tricuspid IE in horses. Two of six reported cases of tricuspid IE7 had recent jugular thrombophlebitis; a third case had an inactive thrombosis related to treatment for an unrelated condition 1 year earlier.4 Permanent IV devices, such as transvenous pacing devices, are rarely used in horses but can predispose to IE.27 IE has also been reported in foals with concurrent septicemia, umbilical infection,7 and osteoarthritis,11 but in most adult cases the route of infection remains unclear.
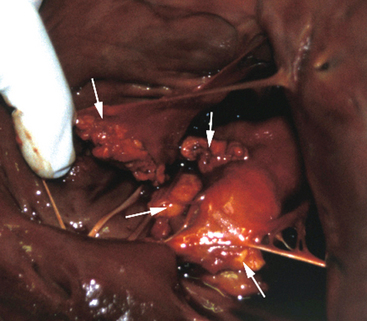
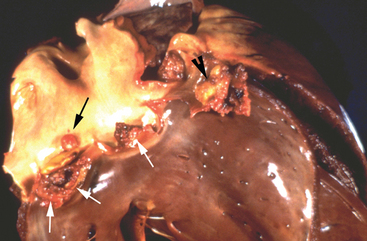
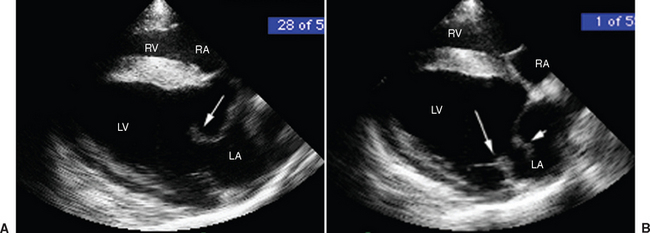
Once a critical mass of bacteria has been deposited on an area of damaged endothelium, vegetations consisting of platelets, fibrin, microorganisms, exopolysaccharides, inflammatory cells, and associated necrotic debris begin to develop.28 Mitral and tricuspid vegetations typically occur on the atrial surface of the valve (Fig. 2-1), whereas aortic vegetations are more likely to develop on the ventricular surface. However, vegetations may occur on any endocardial surface, including the valve leaflets, ventricular or atrial endocardium, and the intimal surface of the great vessels28 (Figs. 2-2 and 2-3). Vegetations usually form at the line of valve closure.21 “Kissing lesions” develop by spreading between adjacent cusps21,28 (Fig. 2-4). IE can extend to involve adjacent structures, such as the chordae tendineae7,12,29 and papillary muscles,28 or can form myocardial abscesses through metastatic infection or direct extension.8,30,31 Infected areas may perforate, leading to defects in the septum or aorta31 or septic pericarditis.28 Valve cusps32 and chordae tendineae7,12,29 can rupture, causing catastrophic regurgitation.
The hemodynamic consequences of IE involve the combined effects of regurgitation and the systemic inflammatory response syndrome (SIRS). Severe mitral regurgitation results in pulmonary hypertension and pulmonary congestion and may lead to right-sided heart failure.33 In acute cases, there may be clinical and radiographic signs of pulmonary edema. Horses that survive the initial episode of acute mitral IE may develop signs of congestive heart failure (CHF), and resultant chronic pulmonary hypertension can lead to pulmonary artery rupture.15 In general, aortic regurgitation appears to be better tolerated in horses than in humans, in whom severe hemodynamic collapse occurs more often with aortic IE or myocarditis than with mitral IE.21 Nevertheless, in equine aortic IE, signs consistent with low cardiac output, left-sided heart failure, and CHF are reported.19,31 Concurrent myocarditis or myocardial abscesses compromise myocardial function further, and arrhythmias are common (Fig. 2-5). Clearly, the more extensive the left-sided valvular pathology, the more severe are the hemodynamic consequences. Regurgitation caused by tricuspid IE is likely to have less direct hemodynamic impact,7 although systemic venous and hepatic congestion can be expected.10
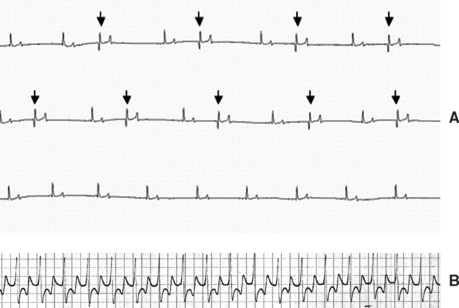
Fig. 2-5 A, Electrocardiogram (ECG) from 13-year-old mixed-breed mare with aortic infective endocarditis (IE) and concurrent myocarditis diagnosed 12 weeks earlier showing numerous isolated ventricular premature depolarizations. The mare’s clinical status had improved considerably since this initial diagnosis, but she continued to have episodes of distress and weakness that were attributed to a paroxysmal ventricular arrhythmia (modified base-apex lead; see also Fig. 2-6). B, ECG from 5-year-old Thoroughbred mare with aortic IE diagnosed 6 days earlier showing an episode of monomorphic ventricular tachycardia that resolved after treatment with magnesium sulfate and lidocaine. One week later, 24-hour ambulatory ECG revealed normal sinus rhythm (base-apex lead; see also Fig. 2-3).
Regardless of the site of the vegetation, bacteremia is likely to induce SIRS and, consequently, distributive shock. The self-amplifying cascade of inflammatory mediators that is triggered in SIRS dysregulates hemodynamic control mechanisms. The pathogenesis of SIRS is described in Chapter 37. In brief, widespread vasodilation produces vascular blood pooling, decreased venous return, and decreased cardiac output.34 This is exacerbated by a direct myocardial suppression, and when these changes are superimposed on mitral or aortic insufficiency, the situation worsens synergistically.35
Once IE is established, in addition to valvular pathology, local infection, bacteremia, and related hemodynamic consequences, embolic complications and immunologic events contribute to disease progression.35,36 Myocarditis results from microabscesses, coronary vasculitis, immune complex deposition, and injury from microbial toxin production.30 Myocardial infarcts,8,9,29 coronary artery thrombosis,6,8 and pulmonary artery thrombosis2,6 may further compromise cardiac function. Embolic pneumonia occurs secondary to tricuspid IE.4,10 Testicular, adrenal, and pancreatic infarcts have been described in a horse with aortic IE and a history of testicular torsion.19 Renal infarcts are found in two thirds of humans who succumb to IE and were present in 8 of 28 horses (28.6%; CI 11.8%-45.3%) at postmortem examination.5,7,9,19 All the equine cases of renal infarct involved IE in the left side of the heart; however, renal infarcts are occasionally associated with right-sided IE in humans, in whom the presumed source of emboli is thrombosed pulmonary vessels resulting from embolic pneumonia.30
Immunologically mediated glomerulonephritis, prerenal azotemia, and disseminated intravascular coagulation are also potential sequelae to IE.4,10,30 The compromised individual with IE is at increased risk of developing acute tubular nephrosis in association with use of antimicrobials, such as the aminogylcosides.30 Lameness and synovial effusion are common. Multifocal synovial distention is generally immunologic in origin;6,12,15,18 however, septic embolism can lead to synovial sepsis, particularly in the digital sheath.4,14 Various forms of neuropathology occur in approximately 30% of humans with IE, about half of whom have associated clinical signs and a high mortality rate.37 These complications are relatively rare in horses, but their prevalence may be underreported (3 of 28 reported cases, 10.7%; CI 0%-22.25%) because of the lack of large, high-quality case series. Meningeal infarcts were described in two horses with IE, one of which had neurologic signs.7 An additional horse with mitral and aortic IE developed unilateral blindness, optic neuritis, uveitis with endophthalmitis, and multifocal suppurative meningoencephalitis in association with Actinobacillus equuli infection.5
Clinical Findings
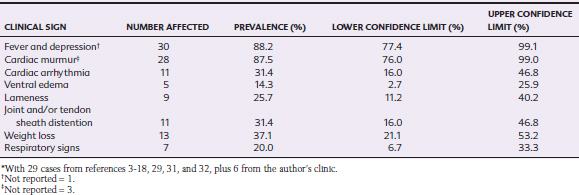
IE has been described in horses ranging from 2 months to 15 years of age, with a median age of 5 years and interquartile range of 10 months to 9 years. Thus, IE appears to be a disease of younger adults, although the demographics of the populations from which these cases were derived are unknown. 3–18,29,31,32 There is no apparent breed predisposition, but the ratio of males to females is 1.85:1, which is similar to that described in humans.22 Fever is the most common presenting sign in horses with IE (Table 2-2).
Cardiac murmurs were present in all animals with left-sided involvement but in only three of six horses with tricuspid IE.4,7,6 In the sole reported equine case of pulmonic IE, no murmur was detected.4,7,14 Therefore, it is important to remember that absence of a cardiac murmur does not exclude a diagnosis of IE.38 Murmurs are most likely to be absent in IE caused by a virulent microorganism that induces rapid, severe disease,30 and interestingly, two of four equine reports include horses that died shortly after the onset of signs.4,7 When present, the murmur of tricuspid regurgitation has its point of maximal intensity (PMI) over the right fourth intercostal space and is usually holosystolic. Mitral regurgitant murmurs are also holosystolic, with their PMI over the left fifth intercostal space. Aortic regurgitant murmurs are holodiastolic, with their PMI over the left fourth intercostal space, high in the axilla. Mitral IE and aortic IE are usually associated with very loud murmurs that radiate over a wide area. Aortic IE murmurs, as with the murmur caused by severe degenerative aortic valve disease, typically have a squeaking or buzzing quality. A mitral regurgitant murmur with a honking quality should raise the suspicion of rupture of one or more chordae tendineae.
Cardiac arrhythmias occur in approximately one third of horses with IE (Table 2-2). Ventricular arrhythmias may include isolated ventricular depolarizations or monomorphic or polymorphic ventricular tachycardias (Fig. 2-5). Episodic collapse or distress can be associated with arrhythmic episodes in both acute and chronic stages. Although no statistical association has been documented, horses with aortic IE appear to be most likely to develop ventricular arrhythmias. In humans, these are the patients most likely to develop coronary thrombosis and myocardial microabscess.30 Supraventricular premature depolarizations7,11,15 and atrial fibrillation11 may occur, particularly in horses with mitral or left-sided atrial mural IE. Signs consistent with CHF, including ventral edema, pleural and peritoneal effusion, and venous congestion, may be detected on presentation or develop as disease progresses.
Lameness is a frequent presenting complaint for horses with IE (Table 2-2). This is often shifting in nature and may be associated with distention of one or more synovial structures.6,12,15,18 IE should be considered as a potential source of hematogenous synovial sepsis.4,14 Right-sided IE generally presents with clinical and radiographic signs relating to embolic pneumonia in humans.38 Other clinical signs reported in horses with IE include ataxia or other neurologic abnormalities, blindness,5 laminitis,7 guttural pouch empyema, sinusitis,26 umbilical infection,29 and physitis.11
Diagnosis
Hematologic and blood biochemical abnormalities are not specific for IE but include leukocytosis (18 of 20 reported cases, 90%; CI 76.9%-100%), hyperfibrinogenemia (13 of 14 reported cases, 93%; CI 79.4%-100%), anemia (12 of 17 reported cases, 52.2%; CI 31.8%-72.6%), and less often, thrombocytopenia (1 of 13 reported cases, 7.1%; CI 0%-20.6%). 3–18,29,31,32 C-reactive protein (CRP), an acute-phase protein that increases in response to infection and inflammation, is considered particularly useful in diagnosis of IE in humans and is used to monitor therapy.39 Suitable alternatives in the horse might be serum amyloid A and fibrinogen concentrations.4 Increases in serum concentrations of creatinine and blood urea nitrogen warrant a guarded prognosis because they may indicate renal infarct.7,19 Measurement of cardiac troponin I40,41 and the cardiac isoenzyme of creatine kinase (CK-MB) can be useful in identifying myocardial lesions. Cardiac arrhythmias should be characterized; ambulatory electrocardiographic (ECG) monitoring may be useful in detecting paroxysmal arrhythmias that are not evident on a short rhythm strip.
The Duke diagnostic criteria for IE in humans, based on laboratory and echocardiographic findings, were developed to categorize patients as definite, possible, or rejected IE cases. Major criteria are (1) persistently positive blood cultures (the specific number of cultures required is defined by the specific organism in question) and (2) echocardiographic evidence of endocardial involvement. Minor criteria include (1) fever, (2) predisposition (e.g., preexisting heart condition), (3) vascular phenomenon (e.g., renal infarcts), (4) immunologic events (e.g., glomerulonephritis, positive rheumatoid factor), (5) positive blood cultures that fall short of the definitions of persistent bacteremia, and (6) suspicious but not definitive echocardiograms. Cases are rejected when (1) a firm alternative diagnosis is made, (2) the clinical signs resolve with antimicrobial therapy in 4 days or less, or (3) pathologic evidence is lacking at surgery or autopsy.42 The Duke criteria are primarily a tool to allow comparison of patient groups in clinical research30 but serve to emphasize the importance of blood culture and echocardiography in the diagnosis of IE. Echocardiography achieves improved sensitivity and equivalent specificity when these criteria are compared with older classification systems based on clinical and laboratory findings alone.43–45 Similarly, in horses the majority of premortem diagnoses of IE are based on echocardiographic findings combined with laboratory findings. 5–8, 10–12, 14–16,18,31
Blood culture is extremely important in the diagnostic evaluation of horses with IE because it may allow identification of a specific microorganism that will help define therapy. In IE there is continuous bacteremia, and therefore timing culture with fever spikes has no advantage. The optimal number of cultures is not known,30 but at least three and ideally five blood samples obtained at hourly intervals using aseptic technique should be submitted. Prior antimicrobial therapy limits the likelihood of positive cultures.20,43,46–48 Microorganisms were identified in 13 of 20 horses (68.4%; CI 47.5%-89.3%) when one to four cultures were submitted (median of two). In all cases, samples were obtained before antimicrobial therapy was initiated by the attending veterinarian, although almost all these horses had received antimicrobial medication before admission to the hospital where they were investigated. Passage of the blood sample through a device designed to remove antimicrobials before inoculation of blood onto culture media may enhance bacterial recovery rates. Cultures should be incubated for a minimum of 4 days before they are classified as negative because prior antimicrobial therapy may delay the growth of microorganisms.20 Additional reasons for negative blood culture include extended course of illness, mural endocarditis, and infection with a fastidious microorganism or an obligate intracellular pathogen.39
The introduction of molecular techniques for the identification of microorganisms has lead to the recognition of a wide spectrum of causal organisms in culture-negative IE in humans,49,50 and similar progress can be expected in veterinary medicine in the future. In such culture-negative infections, however, antimicrobial sensitivity testing is not possible, so the therapeutic advantage of identifying the specific causative organism is lost.50
Echocardiography has a pivotal role in the diagnosis of IE. The presence of an oscillating soft tissue mass attached to the valve cusps, endocardial surfaces of the cardiac chambers, or the intimal surface of the great vessels represents definitive evidence of a vegetation (see Figures 2-3 and 2-4). Many disease processes can cause thickening of the valve cusps, and it can be difficult to distinguish vegetations from other forms of nodular pathology. Recognition of oscillatory movement of a mass independent of movement of the valve confirms that it is a vegetation. In humans the use of transesophageal imaging provides superior resolution and has superior sensitivity to transthoracic echocardiography in detection of vegetations.51 Currently, transesophageal imaging in horses is limited to a small number of veterinary hospitals and requires general anesthesia.52 Because conventional transthoracic echocardiography requires transducers of relatively low frequency, vegetations may go undetected in some cases of equine IE.
Differentiating severe degenerative valvular disease from IE can be difficult. When severe nodular changes are detected in younger animals at low risk of severe degenerative valvular disease, particularly if the nodules are located on the low-pressure aspect of the valve (ventricular surface for aortic valve, atrial surfaces for mitral and tricuspid valves) and are accompanied by clinical and laboratory evidence of infection, a diagnosis of probable IE should be considered, with appropriate treatment instituted (at least until this diagnosis can be rejected after reaching an alternative diagnosis). In the early stages of the disease, vegetations tend to be fairly homogenous (see Figures 2-3 and 2-4), and as they become more organized, they become more echogenic and heterogenous. Additional structural changes may be visible, such as ruptured chordae tendineae, which cause portions of the valve (flail cusp) or chordae to prolapse into the atrium33 (see Figure 2-4).
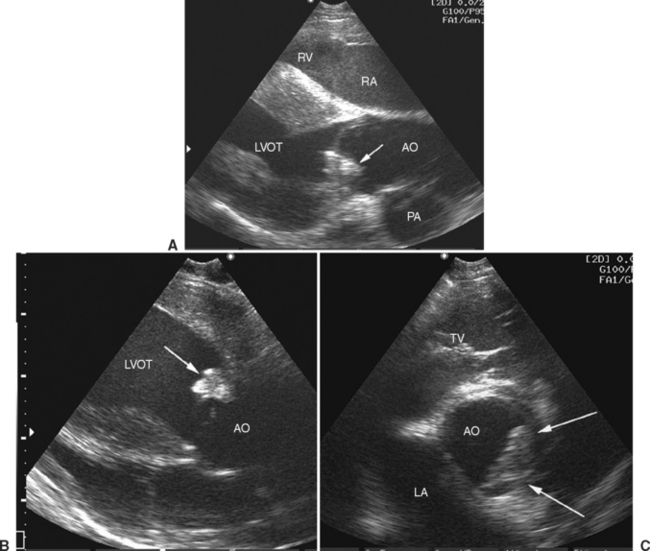
Doppler echocardiography allows identification of regurgitation and permits semiquantitation of its degree,53 which is usually moderate to severe with IE (Fig. 2-6). Jet dimensions provide only a subjective impression of the degree of regurgitation, and these measurements are not very repeatable. Limitations are created by several factors; suboptimal image angulation leads to underestimation of jet size, particularly in the mitral valve, where regurgitant jets are often running at right angles to the image plane, and variation of the image plane is difficult because of anatomic constraints.54 Additional Doppler echocardiographic findings indicative of severe regurgitation include proximal flow convergence and velocity characteristics. Proximal flow convergence is recognized where nonturbulent, retrograde flow can be seen to speed up as it is approaching the regurgitant orifice, represented by bands of color on the proximal aspect of the regurgitating valve (Fig. 2-6). The velocity of flow across a regurgitant orifice or other intracardiac shunt is determined by the pressure difference between the two chambers in question.54 With severe aortic regurgitation, flow early in diastole will have high velocity, but as left ventricular pressures rapidly increase resulting from the entry of the additional regurgitant volume, there will be a rapid deceleration of the regurgitant jet. In the presence of normal left atrial pressures that would be expected with mild mitral regurgitation, regurgitant flow between the left ventricle and left atrium is fast (usually greater than 5 ms−1), whereas with severe regurgitation, the jet velocities are lower. However, accurate flow velocities also depend greatly on the operator, machine, and angle, and these velocities should be interpreted with extreme caution because they have not been validated as indices of severity of regurgitation in the horse. A useful rule of thumb is that if Doppler echocardiographic findings suggest that there is severe regurgitation, this is probably true. However, if Doppler echocardiography fails to demonstrate severe regurgitation in a horse in which clinical findings suggest otherwise, the clinician should remember that the Doppler echocardiographic findings may be misleading.
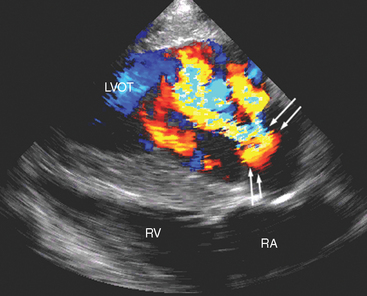
Fig. 2-6 Left long-axis color-flow Doppler echocardiogram from 13-year-old mixed-breed mare with aortic infective endocarditis and concurrent myocarditis of 12 weeks’ duration. Two large (yellow and turquoise) jets of aortic regurgitation occupy most of the left ventricular outflow tract (LVOT), and proximal flow convergence is present (between arrows). RV, Right ventricle; RA, right atrium. (See also Fig. 2-5.)
Two-dimensional and M-mode echocardiography are also useful in assessing the hemodynamic impact of valvular regurgitation.33,55 In long-standing degenerative valve disease, the most accurate assessment of severity is gained by measurement of the diameter of the left ventricle, and these M-mode measurements correlate with heart failure score better than Doppler echocardiographic indices. Because IE is an acute severe condition, however, cardiac remodeling will not have occurred, and often the ventricular dimensions are normal despite the presence of severe regurgitation, although due to volume overload the ventricle may be hyperkinetic with exaggerated movement of the septum and the free wall (Fig. 2-7). Fractional shortening* may be increased provided that myocardial function is maintained, but it may be decreased if there is concurrent myocardial failure. With reduced cardiac output, the diameter of the aortic root may be decreased and the movement of the aortic root depressed on M-mode echocardiography (Fig. 2-7). Dilation of the pulmonary artery is a sensitive indicator of pulmonary hypertension33; it can be identified by comparing the diameter of the pulmonary artery in a long-axis image of the right ventricular outflow tract with the diameter of the aorta in a long-axis image of the left ventricular outflow tract56 (Fig. 2-8). In long-standing cases, signs of ventricular remodeling can be expected. As it enlarges, the left ventricle will typically take on a rounded or globoid shape at the apex, and M-mode measurements of the ventricular dimensions and septal-mitral E-point separation will increase.33,55
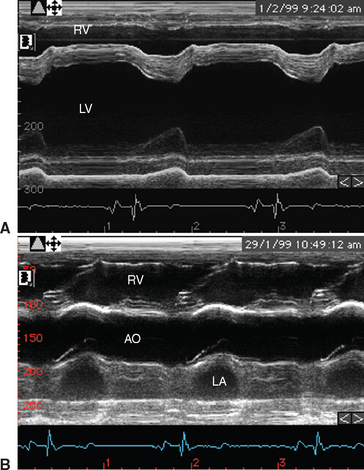
Fig. 2-7 M-mode echocardiograms from 8-year-old polo pony gelding with infective endocarditis diagnosed 2 days earlier. A, Ventricular image demonstrates that although there is no ventricular enlargement, the septal movement is exaggerated because the left ventricle is hyperkinetic. B, Left ventricular outflow image demonstrates flattening of the aortic root secondary to decreased cardiac output. LV, Left ventricle; RV, right ventricle; AO, aorta; LA, left atrium. (See also Figs. 2-4 and 2-8.)
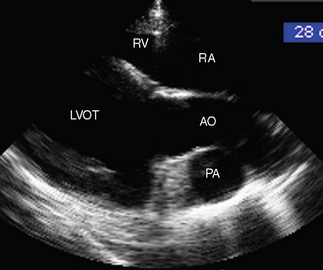
Fig. 2-8 Right long-axis echocardiogram of left ventricular outflow tract (LVOT) from 8-year-old polo pony gelding with infective endocarditis diagnosed 2 days previously. The pulmonary artery (PA) is dilated, and in this view it can be compared directly with the aorta (AO). Note that measurements of the PA are made from the right inflow-outflow view. RA, Right atrium; RV, right ventricle. (See also Figs. 2-4 and 2-7.)
Therapy
The first goal of therapy is sterilization of the vegetations.39,57 Successful treatment of IE requires bactericidal therapy over a prolonged period. It is extremely important to attempt to isolate the organism and determine antimicrobial sensitivity patterns if possible.39 In the absence of specific culture results, broad-spectrum antimicrobial therapy should be instituted. Penicillin and gentamicin are the most common choices,* but previous reports have also described using ampicillin,8 trimethoprim-sulfonamide,3,7 metronidazole,17 oxytetracycline,6 ceftoxamine,11 and rifampicin,14 with no antimicrobial regimen emerging as superior to the others. Bactericidal drugs are preferable to bacteriostatic drugs in this life-threatening bacteremia.39,58 It is difficult to predict the causative organism, although as noted previously, Pasteurella and Actinobacillus species represent about 20% of cases, and Pseudomonas spp. were isolated from about 10%. Consequently, penicillin with an aminoglycoside57 or a fluoroquinolone such as enrofloxacin58 can be predicted to have an appropriate spectrum of activity. Serum bactericidal titers can be used to monitor therapeutic efficacy. Serial dilutions of the patient’s serum, collected at the end of dosing, are tested for their ability to inhibit growth of the bacteria previously isolated from the patient. Serum bactericidal titers of 1:16 or higher have been associated with successful outcomes in human IE.57
Drug efficacy may be compromised by poor penetration of the vegetation, high bacterial numbers and slow growth of deep-seated organisms.57 The diffusion of antimicrobials within vegetations varies; ceftriaxone and penicillin generate a concentration gradient with decreasing levels towards the center, whereas others, such as fluoroquinolones, permeate the vegetation homogenously,59 which, at least theoretically, should confer a therapeutic advantage. However, studies relating specifically to the pharmacokinetics and pharmacodynamics of common antimicrobials used in equine IE are lacking. Rifampin has excellent tissue penetration and should be effective against gram-positive organisms but should not be used in isolation because of concerns over the development of resistance. Rifampin also has potential to induce drug interactions with phenylbutazone and digoxin.58 The clinician should consider the possibility of concurrent renal pathology and prerenal azotemia and should use therapeutic drug monitoring with aminoglycosides to minimize the risk of renal toxicity.
Successful treatment of fungal IE has not been described in horses, although successful treatment of systemic candidiasis in foals with IV amphotericin B and oral fluconazole has been reported.60 In humans, amphotericin B,39 possibly combined with rifampin,61 is used to treat IE caused by candidiasis. Fluconazole is less successful but avoids the nephrotoxic effects of amphotericin.39
Repeat blood cultures do not differentiate between complete and incomplete healing because vegetations may contain deep-seated organisms.28 On the other hand, repeat cultures may be useful in identifying treatment failure.57 In human medicine, serum concentrations of acute-phase CRP are used most often to monitor response to therapy and should begin to decrease within 24 hours of initiating effective therapy.28
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree