CHAPTER 140 Brucellosis in Specialized Livestock
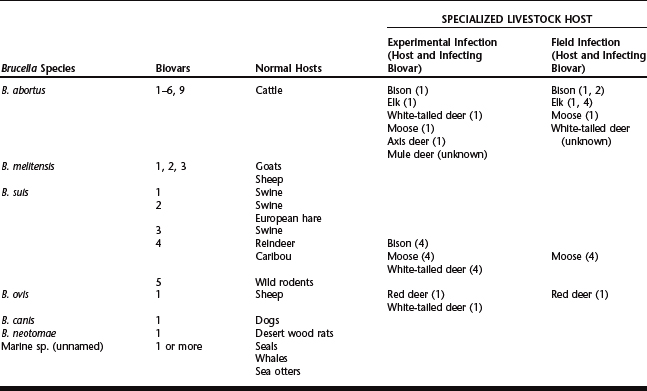
The genus Brucella consists of six formally recognized species of bacteria found in terrestrial mammals (abortus, melitensis, suis, ovis, canis, and neotomae) and a proposed species recently described in marine mammals.1,2 These gram-negative bacteria are the causative agents of brucellosis in animals. A number of species have more than one biologic variant. There are seven recognized biovars of B. abortus, five of B. suis, and three serovars of B. melitensis.3 Two distinct strains of B. canis have been reported, and there is evidence to suggest more than one biovar for the marine isolates.2,4 Brucella ovis and B. neotomae have no reported variants. The preferred hosts for individual species are cattle (B. abortus), goats and sheep (melitensis), sheep (ovis), dogs (canis), and wood rats (neotomae). Brucella suis is notable because it has a wider range of normal hosts than the other species of Brucella and these hosts are associated with specific biovars. Thus, the normal hosts for B. suis biovars 1 and 3 are swine; for biovar 2, swine and the European hare; for biovar 4, reindeer and caribou; and for biovar 5, rodents. The host specificity of Brucella is not always absolute, and it appears that in some cases it is an artifact of normal host range. When a normal and a potential host come in contact through translocation, game farming, extension of agricultural zones into wildlife habitat, or for other reasons, there is an opportunity for interspecific transfer to occur. Examples of this are the spread of B. abortus from cattle to bison and B. ovis from sheep to red deer. The following sections describe the disease by host for bison, elk, reindeer, caribou, red deer, white-tailed deer, mule deer, moose, and other deer species. Table 140-1 lists the species and biovars of the genus Brucella, the normal hosts, and the specialized livestock species that have been infected with brucellosis either in the field or experimentally.
Table 140-1 Normal and Specialized Livestock Hosts for the Species and Biovars of the Genus Brucella
AMERICAN BISON (Bison bison)
Brucellosis in bison probably originated from contact with European cattle in the 19th and early 20th centuries.5 The disease is self-sustaining in bison herds, as evidenced by longstanding infection of relatively isolated populations in Wood Buffalo National Park in Canada, and the greater Yellowstone area of the United States.5,6 Brucella abortus biovar 1 (typical and urease negative) and biovar 2 have been recovered from bison.7,8 Clinical signs, pathogenesis, and epidemiology of bison brucellosis are similar to those seen in cattle, but serologic tests and vaccines developed for use in cattle do not always give equivalent responses in bison and this needs to be considered in diagnosis and control.
Epidemiology
The epidemiology of brucellosis in bison is not well documented, but the similarities of the clinical disease in bison and cattle and the ability of the disease to sustain itself in infected bison herds suggests that epidemiologic aspects of the disease are similar, if not identical, in both species.9 Oral infection is the primary route of infection. Aborted fetuses, placentas, and uterine fluids from infected bison cows can contain high numbers of B. abortus, and persistent vaginal shedding may occur for several weeks post partum. Licking and nosing of aborted materials or the genital regions of postabortion females provides ample opportunity to transmit the disease and these behaviors have been observed in domestic bison. In cattle, persistent shedding of B. abortus may occur after abortion or following subsequent normal calvings, but successive abortions are uncommon. There are no field infection data for bison, but a vaccine strain (B. abortus strain 19) was recovered from a bison cow after her second abortion following vaccination, suggesting that both persistent shedding and successive abortions may occur.10
Venereal transmission rarely occurs in cattle, and infected bulls are considered a negligible risk factor. However, transfer of the organism directly into the uterus via artificial insemination will cause disease.11,12 This is because the vaginal environment is not conducive to survival of Brucella sp., whereas the uterine environment provides optimal growth conditions. Bison bulls infected with B. abortus are similar to bovine bulls and can shed the organism in semen. This is likely not a significant factor in transmission. Two experimentally infected bison bulls shown to be shedding B. abortus in semen were housed with six susceptible bison cows for 10 months. Four cows were successfully bred by one of the bulls and none of the cows or their calves were positive on culture or showed seroconversion.13
In cattle, superovulation, artificial insemination, and embryo transfer have been used to produce disease-free calves from brucellosis-infected cows. Similar procedures have been used with infected donor bison cows to produce calves bacteriologically negative for brucellosis.13 The use of infected cows and bulls for breeding purposes is generally discouraged, but these methods provide an option for the preservation of valuable germ plasm in diseased populations of bison.
Brucella organisms are commonly present in the milk of infected cattle and have been shown to be present in the milk of some experimentally infected bison cows for 3 weeks.9 Live calves born to infected dams are likely to ingest Brucella organisms in milk, but the dose received and the effect of anti-Brucella colostral antibodies are complicating factors. The importance of this route of infection among bison is unknown. Meconium from aborted calves contain Brucella organisms, as do feces from calves nursing infected dams, and these may be a factor in transmission under conditions of close confinement.9
In areas cohabited by bison and cattle, it should be remembered that cattle and bison strains are indistinguishable and that interspecific transmission is possible. Cattle have been infected with Brucella from bison under both field and experimental conditions and B. abortus strain 2308 has been successfully transmitted from infected cattle to noninfected bison cohabiting the same pen.14–16 Experimental infection of six bison with the reindeer strain of brucellosis (B. suis biovar 4) showed that this strain is not pathogenic for bison and that reindeer cohabiting range with bison are unlikely to pose a risk.17
Pathogenesis
The pathogenesis of brucellosis is similar among hosts despite the different species and biovars found in the genus Brucella.18 Infection proceeds through four stages: mucosal infection, infection of local lymph nodes, bacteremia and secondary localization, and reproductive tract localization. Invasion of the mucosa occurs following exposure and elicits an acute inflammatory response in submucosal tissues characterized by increased numbers of mononuclear, polymorphonuclear, and eosinophilic leukocytes; plasma cells; and focal lymphoid aggregates. Brucella sp. are facultative intracellular parasites and likely resist intracellular killing by releasing of 5′-GMP and adenine from their surface, which interferes with degranulation (phagosome-lysosome fusion) and inhibits myeloperoxidase–hydrogen peroxide–halide bacteriocidal activity. Their survival within host leukocytes offers additional protection against other humoral and cell-mediated bacteriocidal responses. Organisms escaping the submucosal response travel to local lymph nodes via lymphatic drainage where they cause enlargement due to lymphoid and reticuloendothelial hyperplasia and the infiltration of inflammatory cells. Survival and multiplication of the organisms in the regional lymph nodes eventually results in spillover into the vascular system and hematogenous spread to other tissues, most notably lymphoid tissues, uterus, and mammary gland in females and testes, epididymis, and accessory sex organs in males. Bones and synovial membranes are less frequently involved and a variety of other tissues and organs can harbor the organism, particularly during the bacteremic phase.
Brucella organisms have an affinity for the placenta, and are found in high numbers in chorionic trophoblasts.18 Chorionic trophoblasts produce a variety of hormones and secretory proteins and appear to provide the factors necessary to stimulate rapid growth of Brucella sp. Localization in the reproductive tract was originally attributed to the presence of erythritol in these tissues, but subsequent studies showed that erythritol was not a critical requirement. Infection may be associated with minimal placentitis or with severe placentitis characterized by widespread destruction of placentomes and fetal membranes. Abortion is a frequent sequela, regardless of the severity of placentitis, and is caused by Brucella lipopolysaccharides and lipid A (endotoxins) present in the uterine environment. The exact mechanism causing abortion is not completely understood and is probably due to a combination of factors. Both placental and fetal progesterone are required to maintain pregnancy in cattle and sheep during the second half of gestation. Virulent strains of Brucella destroy chorionic trophoblasts in vitro, and if this occurs in vivo, it may disrupt the progesterone balance. Bovine and ovine fetuses infected with highly pathogenic strains of Brucella have elevated cortisol levels and this may result in decreased progesterone, increased placental estrogen production, subsequent increases in endometrial prostaglandin (PGF2), and induction of parturition. This fetal effect does not occur consistently with strains of low virulence (RB51, strain 19). Interestingly, abortions have been associated with B. abortus strain 19 vaccination in bison indicating that Brucella strains of low virulence for cattle are not necessarily of low virulence for bison.10
The pathogenesis of mammary gland infection is not well described, but is associated with focally distributed interstitial infiltrates of plasma cells, lymphocytes, macrophages, and variable numbers of neutrophils.18 A variety of pathologic changes associated with inflammatory responses can also occur following localization in the male reproductive tract. Orchitis, epididymitis, and seminal vesiculitis have been described, and lesions may range from severe necrotizing pyogranulomatous orchitis to mild seminal vesiculitis.19
Localization in the joints probably occurs following traumatic injury or endotoxin-mediated changes to capillary permeability resulting in hemorrhage into the joint. Arthritic lesions in bison were characterized by serous arthritis with lymphoplasmacytic infiltrates and hemosiderin-laden macrophages in synovium and proliferation of synovial villi.7 Foci of necrosis and mineralization occurred within the synovium and joint capsule, and there was an infiltrate of epithelioid macrophages, macrophages, and lymphocytes and proliferation of fibroblasts.7
Clinical Signs
Abortions are readily induced by experimental infection of bison with cattle strains of Brucella and abortions due to brucellosis have been described in both wild and ranched bison.9 Based on the limited evidence available, abortions in bison, as in cattle, tend to occur in the last 3 months of pregnancy. Gross lesions of the fetus are seldom observed. Histopathologically, a mild bronchointerstitial pneumonia characterized by neutrophils, mononuclear cells, and degenerate leukocytes in bronchioles and alveoli has been described in aborted fetuses and infected neonates, as have splenic infarction, splenic necrosis, and purulent nephritis.8,20 Retained placentas occur in experimentally infected, vaccinated, and field-infected bison cows.8,9 Metritis in a field-infected bison was associated with a purulent vaginal discharge and necrotic placenta, debris, and purulent exudate in the uterine lumen, all of which were cultured positive for B. abortus, and a recently aborting bison cow had a purulent endometritis and necropurulent placentitis.8,21
Orchitis, epididymitis, and seminal vesiculitis have been described in bison that were serologically or bacteriologically positive for B. abortus.9 Grossly enlarged scrotums were a common clinical sign. Visible lesions ranged from scrotal sacs filled with purulent exudate and caseous testicular remains to thickened epididymal tissue containing foci of yellowish purulent exudate. Gross lesions are not always present, but microscopic lesions in the epididymis, seminal vesicle, and ampulla consisting of lymphoplasmacytic infiltrates with neutrophils in the interstitium and glandular lumens have been described. Based on the limited data, the severity of testicular lesions probably varies with the dose and duration of infection and there is no doubt that reproductive performance is affected in some cases.
B. abortus has been recovered from arthritic joints and hygromas in wild male bison.7 Affected joints may be grossly distended and mobility may be inhibited. Arthritic lesions included loss of articular cartilage with eburnation and lysis of bone, synovial villous hyperplasia, pannus formation, and thickening of joint capsules. Large amounts of translucent viscous yellow fluid containing flecks of debris were present in the joint space and distended tendon sheaths. One bull had a suppurative exudate in the stifle joint. The severity of lesions, and subsequent degree of lameness, likely varies according to the dose and duration of infection.
Diagnosis
Serology
A number of serologic tests are available for use in cattle, and most, if not all, can be used for bison. These tests include the buffered plate agglutination test (BPAT), rapid screening test, brucellosis card test (BCT), standard tube agglutination test (SAT), rivanol test, complement fixation test (CFT), particle concentration fluorescence immunoassay (PCFIA), indirect and competitive enzyme immunoassays (iELISA, cELISA), and most recently, the fluorescence polarization assay (FPA).9,22 The sensitivity and specificity of serologic tests used for bison vary widely, and validation has been hampered by the relatively low number of samples available for study. It has been suggested that serologic responses on some tests are slower to develop in bison than in cattle, and that no serologic test is reliable during the first 8 weeks after infection.16 In general, the tests reliably recognize bison that are positive on culture (sensitivity from 75% to 100%), but do not consistently predict culture results (specificity 36% to 100%).9 The BCT is a readily available and easily conducted field assay and has been used as the sole test to successfully eradicate brucellosis from an infected bison herd. Recent validation work has shown an iELISA, cELISA, and FPA test to have a sensitivity of 96.3%, 96.3%, and 96.3% and a specificity of 97.6%, 94.1%, and 97.6%, respectively, making them the tests of choice.22 The FPA can also distinguish between strain 19 vaccine infection and field infection.22 However, it should be remembered that in the early stages of infection, vaccination and field infection titers may be indistinguishable.
Bacteriology
Recovery of Brucella organisms is required for a definitive diagnosis.3 The success of bacteriologic culture is dependent on the number, type, and quality of samples submitted, the experience of the diagnostic laboratory, and the duration of infection in the animal sampled. Brucella abortus is widely distributed in lymph nodes and organs of infected animals, but not necessarily in all these tissues. In chronic infections, only a few tissues may remain infected and these may contain small numbers of the organism. A large selection of samples is required to maximize the sensitivity of bacteriologic culture. Tissues associated with abortion are important for culture. Samples of choice are fetal stomach contents, fetal lungs, and placenta. Fetal spleen, liver, kidneys, and lymph nodes and vaginal exudates are also good candidates. Milk is a useful sample for bacteriologic culture, but shedding of the organism in milk is often erratic. Mammary tissue provides more reliable results. In the male, semen can be cultured, but infected bulls do not always shed in semen. Bacteriologic culture of testicle and epididymis will give more reliable results, particularly if abnormalities are present. The accessory reproductive glands are of lower priority, but should be sampled if lesions are present. In both sexes, lymph nodes draining the head (retropharyngeal, parotid, mandibular) and inguinal region (internal iliac, supramammary in female; superficial inguinal in male) are tissues of choice for culture, but additional body lymph nodes such as the mediastinal, bronchial, mesenteric, hepatic, suprascapular, prefemoral, and popliteal will enhance the sensitivity of bacteriologic culture. Fluid or suppurative material from affected joints of animals with lameness or joint enlargements should be aseptically collected and cultured. Occasionally the organism will be present in nontypical locations, such as bones or kidneys, and any lesions in animals suspected of having brucellosis should be sampled.
Samples for bacteriologic culture should be chilled to 4° C as soon as possible after collection and processed within 72 hours. If processing will be delayed, samples can be frozen at −20° C and stored for several months. Brucella sp. are slow growing and compete poorly with contaminant bacteria on commonly used media. In most cases, successful isolation requires specialized media and procedures; therefore, samples should be submitted to a laboratory familiar with the isolation of Brucella sp.3 Confirmation and biotyping of suspected Brucella isolates require specialized chemical reagents, bacteriophages, and antisera and are usually performed by a national or international reference laboratory.3 Molecular procedures are being developed for the identification and biotyping of Brucella isolates.
Treatment and Control
Vaccines
Brucella abortus strain 19 is a live vaccine that is commonly used in cattle but offers limited protection against infection and abortion in bison. In addition to this, antibodies produced following vaccination with strain 19 are indistinguishable from those produced following field infection and are a confounding factor in serologic diagnosis. In one study, 58% of pregnant bison aborted following strain 19 vaccination, and one of these bison aborted a strain 19 infected calf during her subsequent pregnancy as well.10 In another study, 61% of vaccinated bison became infected and 33% aborted following challenge with a virulent strain of Brucella (B. abortus strain 2308).10 Female bison vaccinated as 8-month-old calves and challenged with B. abortus strain 2308 during their second trimester of pregnancy had an infection rate of 91% and an abortion rate of 75%.23 Seventy-three percent of pregnant bison vaccinated with strain 19 remained positive on a least one serologic test 10 months after vaccination.10
Brucella abortus strain RB51 is also a live vaccine and was developed from a mutant strain of B. abortus strain 2308 that does not produce the lipopolysaccharide O side chain characteristic of the smooth brucellae. In cattle, this strain protects at least as well as strain 19, does not cause abortion, and does not induce the formation of detectable antibodies on standard serologic tests for brucellosis.24,25 This vaccine has been approved for use in cattle in the United States. Strain RB51 vaccine is less pathogenic for bison than strain 19 vaccine and is cleared by 30 weeks without shedding or lateral transmission following calfhood vaccination.26,27 As in cattle, RB51 in bison stimulates a specific cell-mediated immunity that does not interfere with standard serologic tests for brucellosis.27 Pregnant bison may abort following vaccination using cattle doses, and vaccination of adult bull bison may result in transient shedding of strain RB51 in semen.28,29 In a study using calfhood vaccination with RB51, 15% (4/27) of vaccinated bison aborted following challenge with B. abortus strain 2308 as compared to 62% (4/7) of control subjects.30 There was a significant reduction in the recovery of strain 2308 from the uterus and mammary gland of the RB51-vaccinated animals. In the field, this would reduce the likelihood of both horizontal transmission via fluids associated with abortion or parturition and vertical transmission via milk. Based on these data, it has been suggested that calfhood vaccination with RB51 would be beneficial in reducing transmission of brucellosis among bison. The duration of immunity is unknown, and initial studies on the safety of revaccinating pregnant bison previously vaccinated as calves are not conclusive.31
Zoonotic Potential
All field strains of Brucella from bison and B. abortus strain 19 vaccine are pathogenic for humans. Vaccine strain RB51 is a rough mutant strain and is likely of low virulence for humans. This is based on the low pathogenicity of other rough species (B. ovis and B. canis) for humans and case reports of accidental exposure.32 Until more field data on RB51 are available, it would be prudent to consider it a human pathogen.
NORTH AMERICAN ELK (Cervus elaphus subspp.)
Brucellosis in elk (wapiti) caused by B. abortus originated from contact with infected cattle and bison during the early 20th century.33 Brucella abortus biovars 1 and 4 have been recovered from elk, and clinical signs and pathogenesis of elk brucellosis are similar to those seen in cattle and bison.33 Confinement in pens or population concentrations on managed winter feeding grounds during periods when abortions are occurring provide the necessary conditions for transmission. Currently, brucellosis in elk due to B. abortus occurs only in the wild elk of the greater Yellowstone area of the United States, and control programs using vaccination and feeding ground–habitat modifications are being attempted.33
Epidemiology
Oral exposure is the primary route of infection. Under natural conditions female elk isolate themselves at calving and rapidly consume the products of parturition.34 This strategy appears effective in preventing intraspecific transmission in wild populations. Elk on feedgrounds or in confinement have been observed to smell, lick, and consume products of parturition from other elk, including aborted fetuses and this provides ample opportunity to transmit the disease. Seventeen of 18 unexposed cow elk and 6 of 6 unexposed bull elk became infected following natural exposure to artificially infected elk, indicating that elk are highly susceptible to infection; and 33% to 100% of naturally or experimentally infected elk were shown to either abort or produce nonviable calves, demonstrating a mechanism for the dissemination of large numbers of organisms into the environment.33,35 Three successive abortions were reported in one elk cow.35 In addition to placental and fetal tissue, chorioallantoic fluid is considered a significant source of environmental contamination.35 Vaginal shedding of B. abortus has been reported for up to 17 days following abortion or birth of nonviable calves, and positive vaginal cultures were obtained for up to 9 days following normal parturition in an infected elk cow.35 The duration of infection under natural conditions is unknown. B. abortus has been recovered from experimentally infected elk 56 months after inoculation. Latent infections lasting 23 months (no clinical or serologic evidence of disease) were observed in two elk cows after which they developed serologic titers and aborted, and latent infections probably occur in some calves born to infected dams.35
B. abortus has been recovered from the epididymis, seminal vesicles, ampullae, and semen of experimentally infected elk, but natural breeding of infected bulls to noninfected cows failed to transmit the disease.35 Venereal transmission is not a significant epidemiologic factor in elk, and this is assumed to be for the same reasons as described for bison and cattle.
Brucella organisms were present in the milk of one of five lactating infected elk, indicating that some calves ingest Brucella organisms in milk.35 The dose received and the effect of anti-Brucella colostral antibodies are complicating factors, and the importance of this route of infection among elk is unknown. Meconium from three nonviable calves contained Brucella organisms, but feces from calves nursing infected dams did not, and the authors concluded that environmental contamination from elk calf feces was not important.35
Historical evidence suggests that appropriate calving management under confinement conditions or the opportunity for female elk to exhibit normal isolation behavior at parturition is sufficient to prevent transmission of disease. Brucellosis was confirmed in the elk and bison of the greater Yellowstone area in the early 1900s, and it is generally agreed that it was present for some time before that. Between 1892 and 1967, over 14,000 wild elk from this area were either given or sold to a variety of private, corporate, and government organizations in 38 states and 3 countries.36 It is likely that some of these animals had brucellosis, yet there are no reports of this disease in any of the destination herds. This is likely due to a combination of factors. The number of infected elk transported was probably low. Animals for public display were a novelty at their destination and were closely monitored. Under these conditions it is reasonable to assume that transmission was limited by the prompt removal of aborted fetuses or dead calves, and in many cases, by separation of the valuable pregnant females from the rest of the herd. Elk translocated for restocking or otherwise released under range conditions were able to mimic normal wild behavior at parturition and thus effectively block transmission. In contrast, the wild elk of the greater Yellowstone area have been concentrated in high numbers on managed winter feeding grounds. Under these conditions, detection and isolation of aborting females, effective removal of fetuses, and cleanup of contaminated sites is not possible prior to exposure of other elk, and this appears sufficient to maintain the disease in these herds.
Cattle have been infected with B. abortus from elk under experimental conditions.33 In areas cohabited by bison, cattle, and elk, it should be remembered that the B. abortus strains infecting these species are indistinguishable and that interspecific transmission is possible (although unlikely unless calving or abortion occurs during cohabitation).
Clinical Signs
Abortions in the last 3 months of pregnancy and nonviable calves are the most common clinical signs in both naturally and experimentally infected elk. Fifty-four percent (7 of 13) naturally exposed elk and 61% (40 of 60) experimentally infected elk aborted or had nonviable calves.33,35 Three consecutive abortions were observed in one elk cow; however, the frequency of repeat abortions under field conditions is unknown.35 Gross lesions of the fetus have not been reported. Retained placentas occur in cattle and bison, but have not been observed in elk.35 Infertility in cows is not a common sequela.
Clinical signs and gross lesions of orchitis, epididymitis, and seminal vesiculitis have not been observed in elk, although B. abortus was isolated from the epididymis, seminal vesicles, and ampullae of 4, 7, and 6 of 17 experimentally infected bulls, respectively.35 All tissues of 6 of the 17 infected bulls in this group were negative on culture. There are no confirmed reports of infertility in bull elk associated with B. abortus infection.
Carpal bursitis (hygroma) was the second most frequently observed sign in infected elk.35 It occurred in 12 of 65 elk with experimental infections of at least 1 month duration, although it is usually associated with more chronic infections. Distention was grossly apparent in some cases, and visible only on necropsy in others. This condition was not associated with lameness and often resolved spontaneously. Inflammation of synovial membranes and distention of joint cavities and tendon sheaths, often associated with severe lameness, occurred in 13 of 65 infected elk examined at necropsy.35 Brucella abortus was isolated from the anterior and posterior fetlock, anterior and posterior flexor tendon sheath, and carpal, stifle, and hock joints of these animals.
Diagnosis
Serology
A number of traditional serologic tests designed to detect brucellosis in cattle have been used for elk. The complement fixation test (CFT) and agglutination tests such as the standard plate agglutination test (SPT), buffered plate agglutination test (BPAT), and rivanol test (Riv) have been used successfully to detect infected elk.37 Specificity data for elk are lacking for most of the traditional tests and therefore the incidence of false positive reactions is unknown. The CFT was the most sensitive of these tests, but the anticomplementary activity frequently observed in serum from cervidae can cause inconclusive results. Indirect and competitive enzyme immunoassays (iELISA, cELISA), and the fluorescence polarization assay (FPA) have recently been validated for detection of B. abortus infection in elk and were compared with the CFT.38 The sensitivity and specificity of the iELISA, cELISA, and FPA exceeded 99% for each test, and the FPA was able to correctly distinguish 84% of 55 B. abortus strain 19 vaccinated elk (4 months after vaccination) from infected elk. Test results using a commercially available cELISA kit were 98.6% specific for elk 6 months to 2 years after vaccination with B. abortus strain 19 and 100% sensitive in detecting infected animals.39 However, in elk tested 15 to 43 days after vaccination, specificity dropped to 88%, and the authors suggest that the test not be used within 2 months of strain 19 vaccination.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


