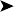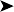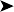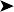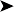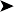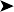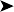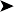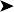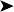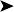Chapter 2 Birds
Case 2.1
Clinical history
The bird had been purchased 18 months previously but had not yet bred. Its appetite was poor.
Clinical diagnosis laboratory
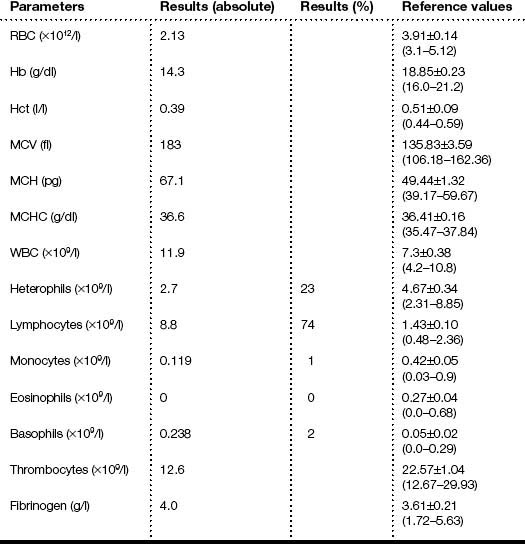
The results of the clinical diagnosis laboratory assays are shown in Tables 2.1 and 2.2.
Table 2.1 Haematology values of the ostrich
| Parameters | Results | Reference values |
|---|---|---|
| RBC (×103/μl) | 1.9 | 1.7–2.17 |
| Hb (g/dl) | 16.1 | 14–17.2 |
| Hct (l/l) | 45 | 41–57 |
| MCV (fl) | 211 | 205–218 |
| MCH (pg) | 79.8 | 76.4–88.4 |
| MCHC (g/dl) | 37.3 | 34.7–41.2 |
| WBC (×109/l) | 60 | 10–24 |
| Heterophils (%) | 33 | 58–89 |
| Lymphocytes (%) | 40 | 12–41 |
| Monocytes (%) | 27 | 0–1 |
| Eosinophils (%) | 0 | 0–4 |
| Basophils (%) | 0 | 0–1 |
Table 2.2 Blood chemistry values of the ostrich
| Analysis | Results | Reference values |
|---|---|---|
| AST (U/l) | 781 | 226–547 |
| Bile acids (μmol/l) | 102 | 2–30 |
| CK (U/l) | 12 412 | 800–6508 |
| Uric acid (μmol/l) | 950 | 59.5–892.2 |
| Urea nitrogen (mmol/l) | 4 | 0–1 |
| Total protein (g/l) | 22 | 24–53 |
| Calcium (mmol/l) | 2.2 | 2.0–3.39 |
| Glucose (mmol/l) | 7.8 | 9.1–18.3 |
Results
 Faecal examination was negative for the presence of endoparasites
Faecal examination was negative for the presence of endoparasites
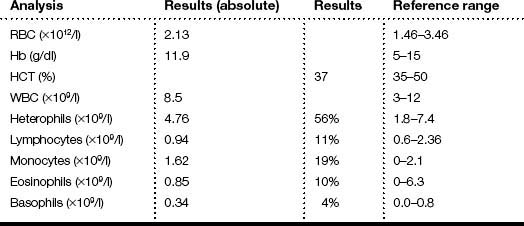
 Haematology analysis showed an elevated WBC with marked monocytosis
Haematology analysis showed an elevated WBC with marked monocytosis
 Blood chemistry analysis showed elevated levels of aspartate aminotransferase (AST), creatine kinase (CK), bile acids, uric acid, and urea nitrogen
Blood chemistry analysis showed elevated levels of aspartate aminotransferase (AST), creatine kinase (CK), bile acids, uric acid, and urea nitrogen
 The cloacal biopsy revealed a granulomatous lesion associated with numerous acid-fast bacilli
The cloacal biopsy revealed a granulomatous lesion associated with numerous acid-fast bacilli
Post-mortem findings
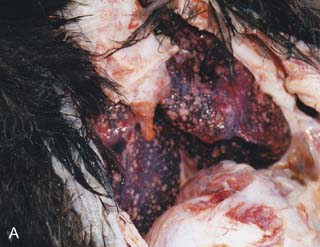
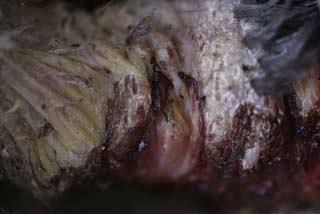
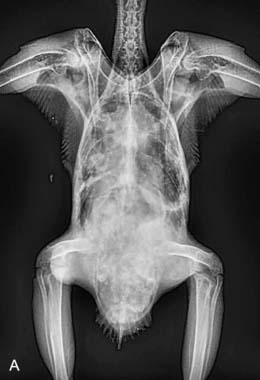
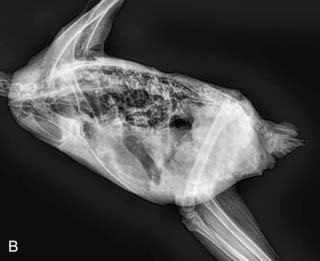
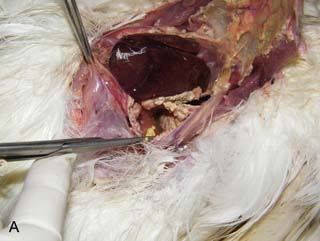
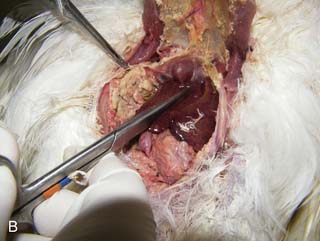
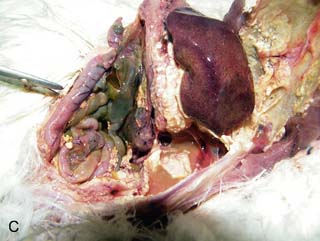
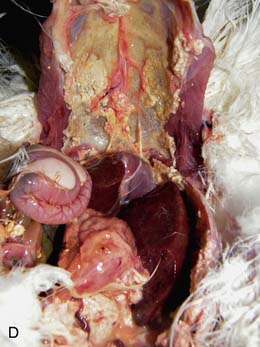
On necropsy, the bird was emaciated with bright yellow subcutaneous and internal body fat. The liver was enlarged with multifocal, densely packed, small white nodules (>5 mm in diameter) on the surface and throughout the parenchyma (Fig. 2.1a, b). Similar nodules of various sizes (2–35 mm) were scattered over the serosa and mucosa of the intestinal tract, protruding into the lumen. Both caecae were covered with a yellow fissured membrane and the lumen contained a clear gelatinous material in which some flocculent material was suspended.
The heart, lungs, air sacs, kidneys and spleen appeared normal.
Summary of post-mortem examination findings
 Liver: enlarged with multifocal, densely packed, small white nodules (>5 mm in diameter) on the surface and throughout the parenchyma
Liver: enlarged with multifocal, densely packed, small white nodules (>5 mm in diameter) on the surface and throughout the parenchyma
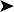 Intestines: similar nodules of various sizes scattered over the serosa and mucosa
Intestines: similar nodules of various sizes scattered over the serosa and mucosa
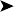 Caecae: covered with a yellow fissured membrane and the lumen contained a clear gelatinous material in which some flocculent material was suspended
Caecae: covered with a yellow fissured membrane and the lumen contained a clear gelatinous material in which some flocculent material was suspended
Laboratory findings
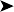 Microbiology: mycobacterial cultures of eight tissue samples (liver, cloaca, caecum and intestine) failed to grow mycobacteria
Microbiology: mycobacterial cultures of eight tissue samples (liver, cloaca, caecum and intestine) failed to grow mycobacteria
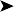 Histology: multiple granulomas were present in the liver, spleen and intestine. Most of the granulomas had a central area of coagulative necrosis surrounded by giant cells, epithelioid macrophages, plasma cells and occasional heterophils. Ziel-Neelsen stains of these lesions revealed masses of slender acid-fast bacilli in the necrotic centres of the granulomas and in the surrounding giant cells and macrophages. No lesions were observed in other tissues
Histology: multiple granulomas were present in the liver, spleen and intestine. Most of the granulomas had a central area of coagulative necrosis surrounded by giant cells, epithelioid macrophages, plasma cells and occasional heterophils. Ziel-Neelsen stains of these lesions revealed masses of slender acid-fast bacilli in the necrotic centres of the granulomas and in the surrounding giant cells and macrophages. No lesions were observed in other tissues
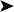 PCR: a multiplex PCR was performed; the results were consistent with Mycobacterium avium
PCR: a multiplex PCR was performed; the results were consistent with Mycobacterium avium
Dawson D.J. Infection with Mycobacterium avium complex in Australian patients with AIDS. Med. J. Aust.. 1990;15(153):466–468.
Doneley R.J.T., Gibson J.A., Thorne D. Mycobacterial infection in an ostrich. Aust. Vet. J.. 1999;77(6):368–370.
Phalen D. Avian mycobacteriosis. Proceedings of the Association of Avian Veterinarians Conference Australian Committee. 1998:71–73.
Reece R.L., Beddome V.D., Barr D.A. Common necropsy findings in captive birds in Victoria, Australia, 1978–1987. J. Zoo Wildl. Med.. 1992;23:301–312.
Sanford S.E., Rehymulla A.J., Josephson G.K.A. Tuberculosis in farmed rhea (Rhea americana. Avian Dis. 1994:193–196.
Shane S.M., Camus A., Strain M.G. Tuberculosis in commercial emus (Dromaius novaehollandiae. Avian Dis. 1993:1172–1176.
Thorel M.F., Huchzermeyer H., Weiss R. Mycobacterium avium infections in animals. Literature review. Vet. Res.. 1997;28:439–447.
Case 2.2
Clinical examination
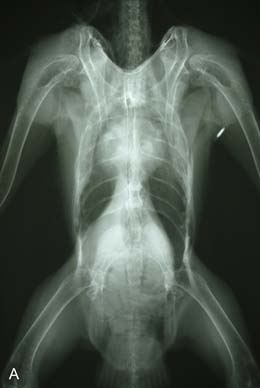
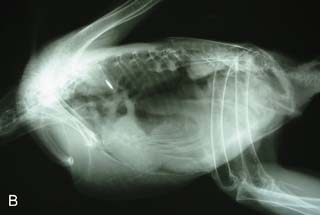
On presentation, the bird was thin with marked loss of pectoral muscle condition and pale mucous membranes. Parasitology crop swabs for wet preparation and faecal samples for direct smear and flotation were performed. Survey radiographs were taken while the bird was under anaesthesia (Fig. 2.2a, b). Blood samples were collected for haematology analysis. Endoscopy examination of the caudal thoracic air sacs and upper digestive tract and trachea was also performed.
Radiology
![]() 1. What is your interpretation of the radiographs in Fig. 2.2a, b?
1. What is your interpretation of the radiographs in Fig. 2.2a, b?
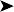 Radiographic findings included a generalized increased radio-opacity of the lung fields and air spaces causing loss of detail over the heart and liver on VD view. Focal radiodensities are apparent in the lung fields and are particularly evident in the right air space adjacent to the liver on the VD view. The proventriculus wall appears thickened and air is evident in the lumen of the proventriculus. The gizzard is small and empty and the spleen is enlarged. The lateral view also reveals increased radio-opacity of the kidneys which are also enlarged.
Radiographic findings included a generalized increased radio-opacity of the lung fields and air spaces causing loss of detail over the heart and liver on VD view. Focal radiodensities are apparent in the lung fields and are particularly evident in the right air space adjacent to the liver on the VD view. The proventriculus wall appears thickened and air is evident in the lumen of the proventriculus. The gizzard is small and empty and the spleen is enlarged. The lateral view also reveals increased radio-opacity of the kidneys which are also enlarged.
Endoscopy
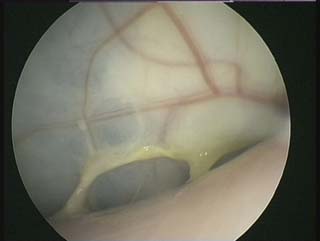
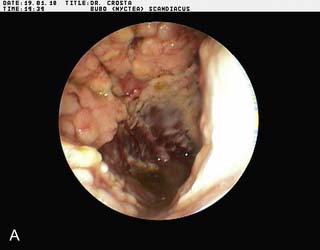
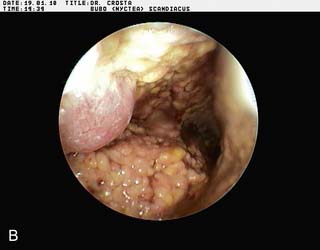
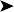 Endoscopy examination of the upper gastrointestinal tract and trachea revealed no abnormal findings. Endoscopy of the caudal thoracic air sacs was conducted (Fig. 2.3). A swab was collected from the caudal thoracic air sacs and submitted for microbiological and cytology investigations (Fig. 2.4).
Endoscopy examination of the upper gastrointestinal tract and trachea revealed no abnormal findings. Endoscopy of the caudal thoracic air sacs was conducted (Fig. 2.3). A swab was collected from the caudal thoracic air sacs and submitted for microbiological and cytology investigations (Fig. 2.4).
Cytology
An endoscopic image and photomicrograph of the cytology preparation collected during endoscopy are shown in Figs 2.3 and 2.4.
![]() 2. What is your interpretation of the endoscopic findings shown in Fig. 2.3? What is your interpretation of the cytology preparation from the air sac?
2. What is your interpretation of the endoscopic findings shown in Fig. 2.3? What is your interpretation of the cytology preparation from the air sac?
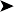 The air sac is opaque and vascularized. Thick yellow fluid is present in the margin of the air sac along with the edge of a yellow plaque
The air sac is opaque and vascularized. Thick yellow fluid is present in the margin of the air sac along with the edge of a yellow plaque
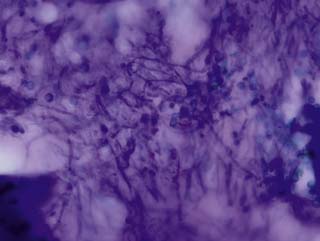
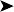 The impression smear from the swab collected from the air sac reveals numerous fungal hyphae and spores
The impression smear from the swab collected from the air sac reveals numerous fungal hyphae and spores
![]() 3. List your differential diagnoses based on the clinical, radiography and endoscopy findings.
3. List your differential diagnoses based on the clinical, radiography and endoscopy findings.
Clinical diagnosis laboratory
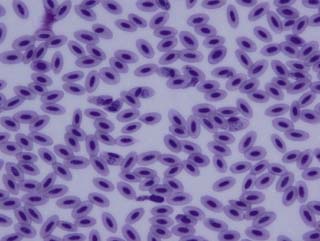
The results of the clinical diagnosis laboratory assays are shown in Table 2.3 and the blood film in Fig. 2.5.
Table 2.3 Haematology values of the African fish eagle
| Parameters | Results | Reference values* |
|---|---|---|
| RBC (×1012/l) | 2.1 | 2.3±0.2 |
| Hb (g/dl) | 3.6 | 12.2±1.1 |
| Hct (l/l) | 10 | 42±4 |
| MCV (fl) | 47.62 | 182.5±19.3 |
| MCH (pg) | 17.1 | 50±1 |
| MCHC (g/l) | 36 | 29.7±0.8 |
| WBC (×109/l) | 3.4 | 14.5±6.8 |
| Heterophils (×109/l) | 2.2 | 10.5±6 |
| Lymphocytes (×109/l) | 1.1 | 2.3±1.4 |
| Monocytes (×109/l) | 0.1 | 0.6±0.6 |
| Eosinophils (×109/l) | 0 | 0.8±0.6 |
| Basophils (×109/l) | 0 | 0.2±0.1 |
* ISIS values are for African fish eagles (n 2–11).
![]() 4. What is your interpretation of the haematology values shown in Table 2.3? What is your interpretation of the blood film in Fig. 2.5?
4. What is your interpretation of the haematology values shown in Table 2.3? What is your interpretation of the blood film in Fig. 2.5?
Other laboratory results
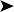 Faecal examination was negative for the presence of endoparasites
Faecal examination was negative for the presence of endoparasites
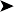 Examination of saline crop swabs was negative for the presence of parasites
Examination of saline crop swabs was negative for the presence of parasites
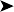 Aspergillus fumigatus was cultured from the swab of the air sac
Aspergillus fumigatus was cultured from the swab of the air sac
Please evaluate the clinical history, Figs 2.2–2.5, the results of the radiography and endoscopic examinations and clinical diagnosis laboratory tests.
Clearly, there are two pathological processes contributing to the morbidity of this bird.
Therapy
If the disease is diagnosed in the early stages, aspergillosis can be successfully treated with oral itraconazole or voriconazole and nebulization with amphotericin B or F10 (Table 2.4). One caution in such a profoundly anaemic bird would be to deal with the malaria first because sometimes the azole antifungal agents can cause anorexia, particularly in debilitated birds.
Table 2.4 Dosage protocols of some antifungal agents in birds
| Drug | Route | Dose |
|---|---|---|
| Itraconazole | PO | Treatment or prevention – 20 mg/kg q24h or 10–15 mg/kg q12h for 30–60 days |
| Voriconazole | PO | 12.5 mg/kg q12h for 30–60 days |
Atkinson C. Avian malaria. In: Atkinson C.T., Thomas N.J., Hunter D.B. Parasitic Diseases of Wild Birds. Ames: Wiley-Blackwell Publishing; 2008:35–53.
Dorrestein G.M. Metabolism, pharmacology and therapy. In: Altman R.B., Clubb S.L., Dorrestein G.M. Avian Medicine and Surgery. Philadelphia: WB Saunders Company; 1997:661–670.
Greiner E.C. Parasites. In: Ritchie B., Harrison G., Harrison L. Avian Medicine: Principles and Application. Lake Worth: Wingers Publishing; 1994:1008–1029.
Hollamby S., Afema–Azikuru J., Sikarskie J.G., et al. Clinical pathology and morphometrics of African fish eagles in Uganda. J. Wildl. Dis.. 2004;40(3):523–532.
Krone O., Cooper J. Parasitic diseases. In: Cooper J., ed. Birds of Prey Health and Disease. Oxford: Blackwell Science; 2002:105–120.
Lichtenberger M. Shock, fluid therapy and CPCR for the avian patient. Proceedings of the 8th European Conference of the Association of Avian Veterinarians. 2005:374–387. Arles, France
Remple D. Intracellular hematozoa of raptors: a review and update. J. Avian Med. Surg.. 2004;18(2):75–88.
Williams R.B. Avian malaria: clinical and chemical pathology of Plasmodium gallinaceum in the domesticated fowl Gallus gallus. Avian Pathol.. 2005;34(1):29–47.
Case 2.3
Physical examination
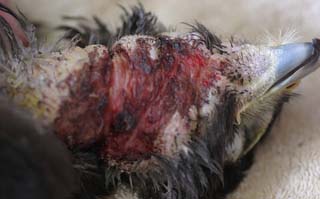
The bird was in good body condition. No abnormality was detected other than a large ulcerated, thickened skin lesion of the ventral neck (Figs 2.6 and 2.7).
![]() 1. What are your differential diagnoses?
1. What are your differential diagnoses?
 Ectoparasites – especially epidermoptid mites
Ectoparasites – especially epidermoptid mites
 Tumour – especially squamous cell carcinoma
Tumour – especially squamous cell carcinoma
 Oral/pharyngeal lesion causing irritation resulting in self-trauma
Oral/pharyngeal lesion causing irritation resulting in self-trauma
The bird was anaesthetized using isoflurane.
Clinical diagnosis examination
Endoscopy examination was carried out of the upper gastrointestinal tract.
Clinical diagnosis laboratory examination
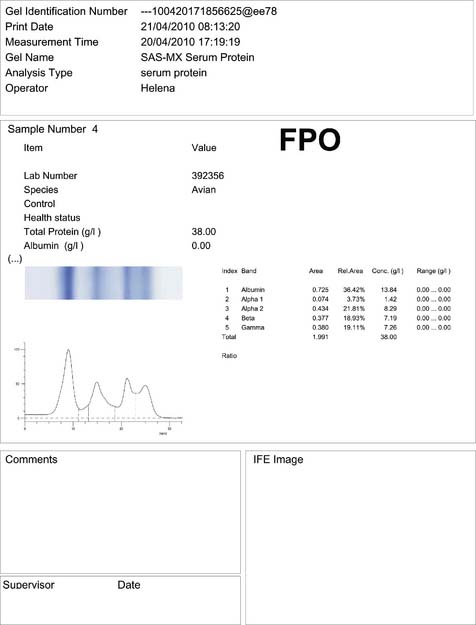
Blood samples were collected for haematology, blood chemistry and protein electrophoresis (Fig. 2.8) analyses (Tables 2.5–2.7). In addition, cytology smears, skin biopsies and bacteriology swabs were collected from the lesion.
Table 2.5 Results of the biochemistry analyses in the Harris’ hawk
| Analysis | Results | Reference ranges |
|---|---|---|
| Total protein (g/l) | 38 | 22–40 |
| Albumin (g/l) | 13 | 9–26 |
| Globulin (g/l) | 25 | 12–29 |
| Albumin:globulin ratio | 0.5 | |
| Total calcium (mmol/l) | 2.55 | 1.78–2.95 |
| Phosphate (mmol/l) | 1.5 | 0.39–3.65 |
| Uric acid (μmol/l) | 348 | 143–980 |
| AST (U/l) | 171 | 118–723 |
| Gamma GT (U/l) | 8 | 1–20 |
| CK (U/l) | 549 | 240–1500 |
Table 2.7 Results of the plasma protein electrophoresis analysis in the Harris’ hawk
| Fraction | Results (g/l) |
|---|---|
| Total protein | 38.0 |
| Pre-albumin | |
| Albumin | 13.84 |
| Alpha 1 globulin | 1.42 |
| Alpha 2 globulin | 8.29 |
| Beta globulin | 7.19 |
| Gamma globulin | 7.26 |
Summary of diagnostic results
 Endoscopy of the oral cavity and oesophagus showed no abnormalities
Endoscopy of the oral cavity and oesophagus showed no abnormalities
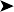 Impression smears of the lesion showed mixed bacteria and a heterophilic response
Impression smears of the lesion showed mixed bacteria and a heterophilic response
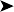 A bacteriological swab from the lesion grew a pure growth of non-haemolytic Escherichia coli sensitive to all routine antibiotics
A bacteriological swab from the lesion grew a pure growth of non-haemolytic Escherichia coli sensitive to all routine antibiotics
![]() 3. What is your interpretation of the blood analyses?
3. What is your interpretation of the blood analyses?
![]() 4. What do you think of the significance of the bacterial isolate?
4. What do you think of the significance of the bacterial isolate?
Chitty J. Raptors: feather and skin diseases. In: Chitty J., Lierz M. BSAVA Manual of Raptors, Pigeons & Passerine Birds. Gloucester: British Small Animal Veterinary Association; 2008:270–277.
Forbes N.A., Cooper J.E., Higgins R.J. Neoplasms of birds of prey. In: Lumeij S., Remple D., Redig P. Raptor Biomedicine. Lake Worth: Zoological Education Network; 2000:127–147.
Lightfoot T., Garner M. Overview of tumors. In: Harrison G., Lightfoot T. Clinical Avian Medicine. Palm Beach: Spix Publishing; 2006:559–572.
Case 2.4
Physical examination
On examination, the hawk was found to be in good body condition, with a well-developed ventral brood patch and a cloacal prolapse as shown in Fig. 2.9.
Clinical diagnosis examination
![]() 1. What are the structures which could be found prolapsed from a cloaca of a female bird?
1. What are the structures which could be found prolapsed from a cloaca of a female bird?
![]() 2. What are the possible causes of cloacal prolapse?
2. What are the possible causes of cloacal prolapse?
![]() 3. What structure is prolapsed in this case?
3. What structure is prolapsed in this case?
![]() 4. What tests should be performed to elicit the cause of prolapse in this case?
4. What tests should be performed to elicit the cause of prolapse in this case?
Summary of diagnosis results
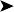 Haematology and biochemistry parameters were all normal, with the exception of an elevated total calcium level (a common feature in egg laying or pre-egg laying female birds)
Haematology and biochemistry parameters were all normal, with the exception of an elevated total calcium level (a common feature in egg laying or pre-egg laying female birds)
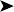 Coelioscopy normal – no indication of peritonitis, further egg progression, enteritis, or cloacitis
Coelioscopy normal – no indication of peritonitis, further egg progression, enteritis, or cloacitis
 Faecal cytology normal, no abnormal bacteria, yeasts or parasites
Faecal cytology normal, no abnormal bacteria, yeasts or parasites
 Clinical examination: the cloacal sphincter is dilated and floppy. The oviductal entrance to the cloaca is inflamed and apparently traumatized
Clinical examination: the cloacal sphincter is dilated and floppy. The oviductal entrance to the cloaca is inflamed and apparently traumatized
![]() 5. List your differential diagnoses.
5. List your differential diagnoses.
Differential diagnoses
 Cloacitis secondary to trauma at egg laying
Cloacitis secondary to trauma at egg laying
 Cloacal sphincter trauma following first egg laying at an advanced age
Cloacal sphincter trauma following first egg laying at an advanced age
 Cloacal sphincter stretching secondary to hypersexuality
Cloacal sphincter stretching secondary to hypersexuality
 The aggression noted towards other humans and egg laying in absence of a mate tends to indicate that the bird has a desired breeding relationship with the owner. Such an abnormal relationship will often result in abnormal egg laying, cloacal sphincter stretching and secondary cloacitis.
The aggression noted towards other humans and egg laying in absence of a mate tends to indicate that the bird has a desired breeding relationship with the owner. Such an abnormal relationship will often result in abnormal egg laying, cloacal sphincter stretching and secondary cloacitis.
Therapy
 Topical treatment for cloacitis
Topical treatment for cloacitis
 Cloacopexy – i.e. after reduction of the cloacal prolapse, placing a cotton bud into the cloaca and pushing as craniad as possible, then fixation either percutaneous or via an abdominal incision, to the ventral abdominal wall, or if possible bilaterally to the last (8th) rib
Cloacopexy – i.e. after reduction of the cloacal prolapse, placing a cotton bud into the cloaca and pushing as craniad as possible, then fixation either percutaneous or via an abdominal incision, to the ventral abdominal wall, or if possible bilaterally to the last (8th) rib
 Cloacoplasty – i.e. reduction of the internal circumference of the cloacal sphincter, by bilateral removal of mucosa and placement of sutures from the external skin surface, through the skin, muscle and mucosa and back to the skin, in order to reduce the cloacal circumference
Cloacoplasty – i.e. reduction of the internal circumference of the cloacal sphincter, by bilateral removal of mucosa and placement of sutures from the external skin surface, through the skin, muscle and mucosa and back to the skin, in order to reduce the cloacal circumference
 On recovery, maintaining the bird in a darkened environment, without exposure to the falconer
On recovery, maintaining the bird in a darkened environment, without exposure to the falconer
Forbes N.A. Soft tissue surgery. In: Samour J., ed. Avian Medicine. second ed. Oxford: Mosby Elsevier Ltd; 2008:163–164.
Forbes N.A. The use of GnRH implants in the treatment of sexually derived behavioural abnormalities in birds. Proceedings of the European Association of Avian Veterinarians. 2009:119–122. Antwerp
Case 2.5
Clinical diagnosis examination
On presentation, the bird had a reduced body condition of 2/5. Survey radiographs were taken while the bird was under anaesthesia (Fig. 2.10a, b).
Clinical diagnosis laboratory examination
Blood samples were collected for haematology, but the client declined blood chemistry. The results of the haematology analysis are shown in Table 2.8.
Film comments
![]() 2. What is your interpretation of the haematology values shown in Table 2.8?
2. What is your interpretation of the haematology values shown in Table 2.8?
After having explained the results, the owner agreed to a coelioscopy, to investigate the abdominal radiodense masses as observed in the survey radiographs (Fig. 2.11a, b).
Summary of clinical diagnosis laboratory results
 Haematology revealed a moderate leucocytosis, a moderate to high heterophilia and heterophil toxicity
Haematology revealed a moderate leucocytosis, a moderate to high heterophilia and heterophil toxicity
 Both Gram staining and cytology confirmed the absence of microorganisms
Both Gram staining and cytology confirmed the absence of microorganisms
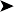 Furthermore, cytology detected large foamy cells with marked anisocytosis and anisokaryosis, and large atypical fibroblasts
Furthermore, cytology detected large foamy cells with marked anisocytosis and anisokaryosis, and large atypical fibroblasts
Please evaluate the clinical history, Figs 2.10a, b, 2.11a, b, the results of the physical examination and clinical diagnosis laboratory tests.
![]() 3. List your differential diagnoses.
3. List your differential diagnoses.
Therapy (Table 2.9)
Unfortunately, after 2 days of treatment and no obvious improvement, the owner opted for euthanasia.
| Ceftriaxone | 100 mg/kg IM bid × 1 week |
| Vitamin ADE | Vitamin A 20 000 IU/kg IM once |
| Fluids/vitamin B complex | Ringer’s lactate 20 ml/kg with 30 mg/kg thiamine SC BID |
| Itraconazole | 10 mg/kg PO BID |
Post-mortem findings
A thorough post-mortem examination was performed (Fig. 2.12a–d). Samples from different organs were collected for histopathology.
![]() 5. What lesions can you observe in the post-mortem photographs?
5. What lesions can you observe in the post-mortem photographs?
Summary of post-mortem examination findings
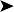 Almost every organ looked “rotten” inside the bird
Almost every organ looked “rotten” inside the bird
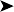 Liver: marbled, but without specific focal foci
Liver: marbled, but without specific focal foci
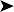 Coelomic cavity: presence of multiple small, caseous masses, possibly granulomatous in origin, infiltrating several organs and serosa
Coelomic cavity: presence of multiple small, caseous masses, possibly granulomatous in origin, infiltrating several organs and serosa
 Intestine: no gross changes, apparently only involved mechanically
Intestine: no gross changes, apparently only involved mechanically
Laboratory findings
Histology
 Spongy tissue with trabecula that often looked necrotic. In the spaces between the mesh large macrophages and granulocytes, but in many of these “chambers” basophilic crystal-like cigar to round structures that were small (diameter 2–3 μm) and irregular
Spongy tissue with trabecula that often looked necrotic. In the spaces between the mesh large macrophages and granulocytes, but in many of these “chambers” basophilic crystal-like cigar to round structures that were small (diameter 2–3 μm) and irregular
 In other places it looked more like a xanthoma, including cholesterol clefts
In other places it looked more like a xanthoma, including cholesterol clefts
 Liver reactive with mixed cellular periportal and sinusoidal infiltrates
Liver reactive with mixed cellular periportal and sinusoidal infiltrates
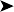 In the lungs locally parabronchi filled with the large spongy macrophages seen in the tumour and also basophilic changes (metastasis or inhalation?)
In the lungs locally parabronchi filled with the large spongy macrophages seen in the tumour and also basophilic changes (metastasis or inhalation?)
Cooper J.E. Miscellaneous and emerging diseases. In Birds of Prey: Health and Disease, third ed., Oxford: Blackwell Publishing; 2002:200–201.
Forbes N.A. Raptor medicine. Seminars in Avian and Exotic Pet Medicine. 2000;9:197–203.
Forbes N.A., Cooper J.E., Higgins R.J. Neoplasm of birds of prey. In: Lumeij J.T., Remple J.D., Redig P.T. Raptor Biomedicine III. Lake Worth, FL: Zoological Education Network; 2000:127–146.
Garner R.R. Overview of tumors: section II – a retrospective study of case submissions to a specialty diagnostic service. In: Harrison G.J., Lightfoot T. Clinical Avian Medicine. Palm Beach: Spix Publishing, Inc; 2006:5665–5671.
Latimer S.K. Oncology. In: Ritchie B.W., Harrison G.J., Harrison L.R. Avian Medicine: Principle and Application. Lake Worth: Wingers Publishing, Inc; 1994:640–672.
Lightfoot T.L. Overview of tumors: section I – clinical avian neoplasia and oncology. In: Harrison G.J., Lightfoot T. Clinical Avian Medicine. Palm Beach: Spix Publishing, Inc; 2006:560–565.
Raynor P.L., Kollias G.V., Krook L. Periosseous xanthogranulomatosis in a fledgling great horned owl (Bubo virginianus. J. Avian Med. Surg.. 1999;13(4):269–274.
Rettenmund C., Sladky K.K., Rodriguez D. Pulmonary carcinoma in a great horned owl (Bubo virginianus. J. Zoo Wildl. Med.. 2010;41(1):77–82.
Ridgway R.L. Oral xanthoma in a budgerigar (Melopsittacus undulatus): a case report. Vet. Med. Small Anim. Clin.. 1977;72(2):266–267.
Schmidt R.E., Reavill D.R., Phalen D.N. Pathology of Pet and Aviary Birds. Ames: Blackwell Publishing; 2008.
Case 2.6
Clinical diagnosis examination
Survey radiographs were taken while the bird was under anaesthesia (Fig. 2.13a, b). Endoscopy examination of the upper digestive tract and trachea was also performed.
Clinical diagnosis laboratory examination
The results of the clinical diagnosis laboratory assays are shown in Tables 2.10–2.12.
Table 2.11 Blood chemistry values of the gyr falcon
| Analysis | Results | Reference values |
|---|---|---|
| Albumin (g/l) | 12.4 | 11.8 (1.7) |
| ALKP (U/l) | 97.54 | – |
| Amylase (U/l) | 60.1 | 86 (116) |
| Bile acids (μmol/l) | 460 | – |
| Bilirubin (μmol/l) | 17.44 | 4.6 (1.7) |
| Calcium (mmol/l) | 2.13 | 2.3 (0.27) |
| Cholesterol (mmol/l) | 2.15 | 5.44 (1.03) |
| Creatinine (μmol/l) | 116.69 | 38 (14) |
| CK (U/l) | 1066.4 | – |
| GGT (U/l) | 5.73 | – |
| AST (U/l) | 418.4 | 149 (110) |
| ALT (U/l) | 221.6 | 135 (125) |
| Glucose (mmol/l) | 16.21 | 20.4 (1.7) |
| Iron (μmol/l) | 12.27 | – |
| LDH (U/l) | 7335.5 | 1917 (879) |
| Phosphorus (mmol/l) | 1.70 | 1.52 (0.40) |
| Total protein (g/l) | 32.0 | 25.0 (8.7) |
| Total urea (mmol/l) | 1.73 | 3.6 (2.2) |
| Uric acid (μmol/l) | 1225 | 370 (170) |
Table 2.12 Plasma protein electrophoresis of the gyr falcon
| Parameters | Fractions (%) | Concentrations (g/l) |
|---|---|---|
| Total protein | 32.0 | |
| Albumin | 12.7 | 4.06 |
| Alpha 1 globulins | 8.0 | 2.56 |
| Alpha 2 globulins | 22.0 | 7.04 |
| Beta globulins | 36.0 | 11.52 |
| Gamma globulins | 21.3 | 6.82 |
| A:G ratio | 0.15 |
Summary of diagnostic results
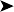 Radiographic findings included mainly an enlarged liver shadow and a slightly distended gizzard
Radiographic findings included mainly an enlarged liver shadow and a slightly distended gizzard
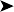 Endoscopy examination of the upper gastrointestinal tract and trachea revealed no abnormal findings
Endoscopy examination of the upper gastrointestinal tract and trachea revealed no abnormal findings
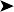 Faecal examination showed negative result for the presence of endoparasites
Faecal examination showed negative result for the presence of endoparasites
 Haematology analysis showed low RBC, low Hb and low Hct. The WBC was slightly elevated with marked lymphocytosis
Haematology analysis showed low RBC, low Hb and low Hct. The WBC was slightly elevated with marked lymphocytosis
 Blood chemistry analysis showed elevated levels of bile acids, CK, glutamic oxaloacetic transaminase (GOT), glutamic pyruvic transaminase (GPT), lactate dehydrogenase (LDH) and uric acid
Blood chemistry analysis showed elevated levels of bile acids, CK, glutamic oxaloacetic transaminase (GOT), glutamic pyruvic transaminase (GPT), lactate dehydrogenase (LDH) and uric acid
 Plasma protein electrophoresis showed low albumin, elevated globulins and low albumin:globulin ratio
Plasma protein electrophoresis showed low albumin, elevated globulins and low albumin:globulin ratio
Please evaluate the clinical history, Fig. 2.13a, b, the results of the physical examination and clinical and laboratory diagnostic tests.
![]() 4. List your differential diagnoses.
4. List your differential diagnoses.
Therapy
The falcon was placed on the therapeutic management shown in Table 2.13.
| Marbofloxacin 10% | 15 mg/kg IM SID × 1 week |
| Vitamin ADEC | To provide Vitamin A 20 000 IU/kg IM, once |
| Vitamin B complex | To provide thiamine 30 mg/kg IM, once |
| Fluids | Ringer’s lactate 20 ml/kg SC BID |
| Forced feeding | Mixture of ground whole quail, ground quail liver, Spark liquid concentrate™ and water, 30–40 ml TID |
Post-mortem examination
The falcon continued deteriorating despite support therapy and died on the fourth day of admission. A thorough post-mortem examination was performed (Fig. 2.14). Samples from different organs were collected for microbiology, histopathology and virus isolation.
![]() 6. What lesions can you observe in the post-mortem photograph?
6. What lesions can you observe in the post-mortem photograph?
Summary of post-mortem examination findings
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree