Fig. 5.1
Actogram of locomotor activity from a single zebra finch, Taeniopygia guttata. The top bars indicate the times during which lights are off (black) vs. on (white). These are plotted in a 48 h time span in order to “double-plot” the data. The time off lights on, ϕ e, indicated by the arrow, is a phase reference determined by the investigator. The internal phase, ϕ i, is determined to be the activity onset. The relationship between ϕ i and ϕ e is called ψ ie. This relationship may change depending on the time of year and physiological condition of the bird. The internal period, τ, is indicated here as the average interval between activity onsets
Similarly, annual environmental cycles correspond to circannual rhythms expressed by many organisms when placed experimentally in constant photoperiods of 12 h of light and 12 h of dark (LD12:12) or another constant photoperiod (Gwinner 1989, 2003). Under these conditions many organisms, including birds, will express cycles of approximately 365 days. These in turn are believed to be entrained to the annual cycle by changes in photoperiod (Gwinner 1989) and/or a physiological proxy, such as the duration of the hormone melatonin (see below). Circannual cycles are not as well understood as are circadian rhythms, but they are likely linked physiologically (Gwinner and Brandstätter 2001; Gwinner et al. 1997).
The role of the circadian clock in annual cycles has been known for some time (Bünning 1969; Follett et al. 1992; Konishi et al. 1987; Menaker and Eskin 1967; Rowan 1926). In many species of birds, exposure to photoperiods of longer than 11.5 h/day results in the rapid induction of the hypothalamo–hypophysial–gonad axis, causing development and growth of testes and ovarian follicles. Although there are differences among species of birds and between birds and other taxa, neither the absolute length of the photoperiod, length of the scotoperiod (the duration of the dark phase), or their ratio is the proximal causes of gonadal induction. Rather, it is the circadian ϕ at which light impinges on photoreceptive elements that causes reproductive changes. For example, male Japanese quail, Coturnix japonica, and white-crowned sparrows, Zonotrichia leucophys, which are maintained in LD 6:18 will exhibit regressed testes. However, if the last hour of the 6 h. photoperiod is extended each long night to a specific “photoinducible phase,” ϕ pi, usually 11–12 h following the onset of the short photoperiod, reproductive activity is commenced. Thus, birds exposed to LD 6:18 or L5: D1: L1: D17 (a single 1 h light pulse interrupting the night) will retain regressed gonads, but if the 1 h pulse occurs 5 h later (L5:D6:L1:D12), gonads will recrudesce (Follett et al. 1974; Menaker and Eskin 1967; Schleussner and Gwinner 1989; Sharp 2005; Yoshimura 2010). The same total amount of light (and dark) is present for each 24 h, but the effect is dramatically different. It is the timing of light coinciding with an internal process that induces or prevents reproductive activity, suggesting a circadian clock underlies photoperiodic time measurement. Nanda and Hamner (1958) had shown this to be the case in plants through an elegant series of experiments in which soy bean plants exposed to a 6 h photoperiod coupled with scotoperiods of varying lengths that were multiples of a 24 h cycle (e.g., LD6:18; LD6:42 or LD6:66) did not flower. However, when plants were exposed to 6 h photoperiods followed by scotoperiods of lengths that did not resonate with 24 h (e.g., LD6:6; LD6:30 or LD6:54) flowering was observed. Similar studies in several species of birds showed that seasonal changes in both gonadal recrudescence and regression are regulated by a circadian clock (Follett and Pearce-Kelly 1991; Follett et al. 1992; Kumar et al. 1996).
These and other observations have led to two competing models for a role of circadian clocks in photoperiodic time measurement. In one, the “external coincidence model” proposed by Bünning (1969) suggests that light has two complementary roles. First, light entrains the circadian clock with a stable ψ ie such that the photoinducible phase, ϕ pi, is also maintained with a stable ψ approximately 11.5 h following the beginning of the photoperiod (lights on). When light coincides with the ϕ pi either because the photoperiod is long under natural conditions or through exposure to an experimental light pulse, the reproductive axis is induced. The competing notion, the “internal coincidence model” proposed by Pittendrigh (cf. Pittendrigh 1993), stems from the observation that many circadian systems behave as if they are composed of at least two oscillators, one entrained to dawn and the other to dusk. In the internal coincidence model the phase relationship between the dawn oscillator to the dusk oscillator, ψ dawndusk, induces seasonal changes in reproduction. As we will see below, each of these models has support from different experimental systems in birds. However, until specific structures and/or molecules become associated with these models, they are essentially untestable at the physiological level.
5.3 Molecular Genetics of Circadian Clock Function
Circadian rhythms are regulated by a highly conserved set of genes, collectively called “clock genes,” whose products are believed to dynamically interact to elicit rhythmic patterns of transcription, translation, biochemical and physiological processes, and behavior (Fig. 5.2) (Bell-Pedersen et al. 2005; Eckel-Mahan and Sassone-Corsi 2013). In animals ranging from Drosophila to humans, the central core of this gene network can be broadly characterized as “positive elements” clock and bmal1 and “negative elements” Period 1 (Per1), period 2 (per2), period 3 (per3), and the cryptochromes cryptochrome 1 (cry1) and cryptochrome 2 (cry2). In contrast to mammals, birds do not express a per1 and have been shown to only express only per2 and per3 (Bailey et al. 2003, 2004; Yasuo et al. 2004; Yasuo and Yoshimura 2009; Yoshimura et al. 2000). Clock and bmal1 are transcribed and then translated in the cytoplasm, where they dimerize and reenter the nucleus and activate transcription of the negative elements through the activation of E-box promoter elements. The pers and crys in turn are transcribed and translated in the cytoplasm, where the PER proteins are targeted for proteosomal proteolysis by a series of protein kinases, most notably casein kinase 1ε (CK11ε) and CK11δ. This process slows the accumulation of the cytoplasmic PER and thereby increases the period of the molecular cycle. In the cytoplasm, PER and CRY proteins form oligomers that reenter the nucleus and interfere with the CLOCK/BMAL1-mediated activation. A secondary cycle involving two genes containing E-box promoters, Reverbα and rorα, amplify the cycle by activating and inhibiting bmal1 transcription respectively. Disruption and/or knock-out of these genes’ action has profound effects on the expression of circadian rhythms in animals in which these technologies are possible (i.e., mice and Drosophila) ranging from changes in period to arrhythmicity.
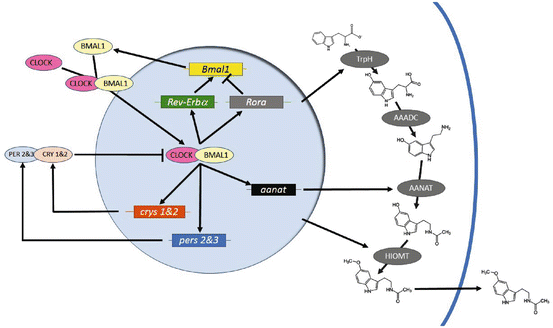

Fig. 5.2
Schematic of the molecular clockworks regulating circadian patterns of melatonin biosynthesis in a pinealocyte or retinal photoreceptor. Positive elements CLOCK and BMAL1 enter the nucleus and activate expression of genes whose promoters contain an E-Box. Among these are the negative elements period 2 & 3 (pers 2 & 3) and cryptochromes 1 & 2 (crys 1 & 2), Rev-Erbα and Rora, which form a secondary loop regulating Bmal1 transcription, and output, clock-controlled genes such as arylalkylamine-N-actyltransferase (aanat). The pers and crys are translated, form heterodimers with other components, such as the casein kinases, and reenter the nucleus to interfere with CLOCK/BMAL1 activation. Melatonin biosynthesis pathways are indicated on the right. Amino acid tryptophan is converted to 5-hydroxytryptophan by tryptophan hydroxylase (TrpH). Aromatic amino acid decarboxylase (AAADC) then converts 5-hydroxytryptophan to 5-hydroxytryptamine (5HT; serotonin), Then, during the night, AANAT converts 5HT to N-acetylserotonin, a substrate for hydroxyindole-O-methyltransferase (HIOMT), which produces melatonin itself. Presumably, melatonin diffuses out of the cell at this time, although a release mechanism may exist
Unfortunately, these technologies are not routinely available in birds just yet. However, there are several observations that tentatively link clock gene expression with avian rhythmic behavior. Noting population studies in human populations of single nucleotide polymorphisms (SNP) in the clock gene’s 3′-UTR have revealed differences in times of sleep and sleep duration (Steinmeyer et al. 2012), have shown weak (5.9 %) to moderate (46.7 %) associations between awakening time of blue tits, Cyanistes caeruleus, with single nucleotide polymorphisms (SNPs) in period 2 and CK1ε. However, these studies were conducted in free-living tits using radio frequency transponders under seminatural conditions, not under DD or dimLL, so that these effects cannot distinguish circadian clock effects from light-induced effects or effects on homeostatic regulation of sleep. Other studies have focused on allelic differences in C-terminal polyglutamine repeats in the CLOCK protein in breeding and migratory patterns of barn swallows, Hirundo rustica, suggesting negative selection on “deviant” genotypes (Caprioli et al. 2012). However, other studies in several different swallow species in the genus Tachycineta failed to show similar associations (Dor et al. 2012).
5.4 Extraocular Photoreception
In addition to photoreceptors in the lateral eyes shared by all vertebrate classes, it has been known for some time that non-mammalian vertebrates express functional photopigments within the brain that are critical for entrainment of both circadian rhythms and circannual cycles. In birds, these reside in the pineal gland, the preoptic area, the lateral septum, and the tuberal hypothalamus. Early studies by Benoit in the 1930s showed that domestic ducks, Anas platyrhynchos, that had been blinded (enucleated) continued to exhibit reproductive responses to changing photoperiod (Benoit and Assenmacher 1954), but work by Menaker and colleagues in the 1960s and 1970s in passerine birds clearly showed that the eyes are not necessary for circadian entrainment (Masuda et al. 1994; McMillan 1970) or seasonal control of reproduction (Menaker 1968; Menaker and Keatts 1968; Menaker and Underwood 1976; Underwood and Menaker 1970). In a classic series of experiments, Menaker’s group demonstrated that enucleated house sparrows could entrain to a series of LD cycles of dimmer and dimmer illuminances (Menaker et al. 1970). Once birds were no longer capable of entrainment, they showed that the responsible photoreceptor resided inside the head by simply plucking feathers, and entrainment was reinstated. They then blocked entrainment by injecting India ink beneath the scalp (Menaker 1968).
Subsequent research has now identified at least four distinct structures within the brain that are functionally photoreceptive, containing several opsin-based photopigments and photoisomerases (Bailey and Cassone 2005; Foster and Hankins 2002; Li and Kuenzel 2008; Masuda et al. 1994). These include the pineal gland, which expresses a pineal-specific opsin, pinopsin (Bailey et al. 2003; Masuda et al. 1994; Max et al. 1995; Okano et al. 1994), as well as melanopsin (OPN4) (Bailey and Cassone 2005; Bailey et al. 2003; Chaurasia et al. 2005); and iodopsin (OPN1) (Bailey et al. 2003; (Masuda et al. 1994) and whose photoreceptive function will be discussed further below. In addition, neurons within the preoptic area express VA (vertebrate ancient) opsin (Davies et al. 2010, 2012; Soni and Foster 1997) and project to the tuberal hypothalamus, while the tuberal hypothalamus expresses a plethora of photoreceptive cells that appear to be divergent among avian species. For example, in domestic turkeys, Meleagris gallopavo, melanopsin-expressing cells in the premammillary nucleus (PMM) in the dorsal tuberal hypothalamus project directly to the median eminence (Kang et al. 2007, 2010; Kosonsiriluk et al. 2013). In Japanese quail, Coturnix coturnix, CSF-contacting neurons in the mediobasal hypothalamus (MBH), which may be homologous to the PMM, express both OPN4 and neuropsin (OPN5) (Nakane and Yoshimura 2010; Nakane et al. 2010). In house sparrows, neurons within the arcuate nucleus express rhodopsin (OPN2) itself, in addition to OPN4 and OPN5 (Foster et al. 1985; Wang and Wingfield 2011). While it is not clear whether or which opsin-based photopigments are expressed in the lateral septal organ, illumination of this area of cerebrospinal fluid contacting neurons elicits a physiological response (Li et al. 2004; Li and Kuenzel 2008). Further, it is not clear whether each of these photoreceptive organs and/or their photopigments subserve mutually exclusive physiological processes or whether these overlap in their functions. In addition to opsin-based photopigments, all animals express flavin-based cryptochromes (Van Gelder 2001). While cryptochrome is the major photopigment responsible for photoentrainment in Drosophila (Emery et al. 1998), the multiple cryptochromes expressed by vertebrates have not been established as photoresponsive molecules. Even so, in birds, the role of cryptochromes in celestial orientation is light dependent (see below).
5.5 The Pineal Gland Is a Master Pacemaker for Avian Circadian Clocks
Searching for the location of the intracranial, extraretinal photoreceptors, Gaston and Menaker (1968) surgically removed the pineal gland (PINX) from house sparrows. While the birds retained their ability to entrain to LD, they became arrhythmic when placed in DD, demonstrating that the pineal gland is necessary for self-sustained circadian rhythmicity. However, the data also showed that the pineal gland is part of a system of circadian clock components, since PINX sparrows could anticipate the time of lights on in an LD cycle and because birds only gradually became arrhythmic over 5–15 days following transfer from LD to DD. Further, the effect of PINX is not universal among avian species. PINX of European starlings, Sturnus vulgaris, results in a range of behavioral changes ranging from arrhythmicity akin to those seen in house sparrows to slight disruption of behavioral locomotor rhythmicity (Gwinner and Brandstätter 2001). Circadian rhythms of locomotor behavior in columbiform and galliform birds are little or not affected at all by PINX (Ebihara et al. 1984; Underwood and Siopes 1984).
Even so, the pineal gland represents both the capacity for rhythmicity and time of day. In an elegant experiment, Zimmerman and Menaker (1979) transplanted pineal glands from two groups of house sparrows into the anterior chambers of the eye of PINX, arrhythmic sparrows maintained in DD. The first group of donor birds were entrained to an early LD cycle, with lights on at midnight, while the second set of donors were entrained to a late LD cycle, with lights on at 11 a.m. Transplantation restored circadian rhythms to both groups of recipients within 1 day. Moreover, birds that received pineal glands from early donors, exhibited an early ϕ i while the recipients of late donor pineal glands exhibited a late ϕ i. Thus, the pineal gland is not only necessary for circadian rhythmicity in these birds, but it contains a correlate that confers time of day to recipient birds.
Importantly, the data also suggested that the pineal gland must affect behavior through the secretion of a hormone, because 1 day is not thought to be sufficient for re-innervation of target tissues, wherever they are. That hormone was known even then to be the indoleamine melatonin from earlier work of Lerner and later of Axelrod, Klein, and their co-workers (cf. Klein et al. 1997), who explored the biochemical basis for melatonin biosynthesis in the pineal gland of the chick, Gallus gallus domesticus. Research from a large number of investigators have shown that pinealocytes, the photoreceptive, secretory cells of the avian pineal gland, take up the amino acid tryptophan, which is converted to 5-hydroxytryptophan by tryptophan hydroxylase (TrH; EC 1.14.16.4) (Chong et al. 2000) and then decarboxylated to produce serotonin (5HT) by aromatic L-amino acid decarboxylase (AAADC; EC 4.1.1.28). During the night in LD and subjective night in DD, 5HT is converted to N-acetylserotonin (NAS) by arylalkylamine (or serotonin)-N-acetyltransferase (AANAT; EC 2.3.1.87) (Bernard et al. 1997b). NAS is then converted to melatonin by hydroxyindole-O-methyltransferase (HIOMT; EC 2.1.1.4) (Voisin et al. 1992). The genes encoding each of these enzymes have been isolated, cloned, and sequenced in several avian species. In chick, at least, TrH, AANAT and HIOMT are regulated by both the molecular clockworks within the pinealocytes and directly by light at the transcriptional, translational, and post-translational levels, so that the enzymatic regulation of pineal melatonin is a dynamic, rhythmic process (Klein et al. 1997).
Avian pineal glands contain the circadian clockworks and photoreceptors to generate circadian patterns of melatonin biosynthesis in vitro as well as in vivo, which can be entrained to LD cycles directly (Binkley et al. 1977a). Pineal tissue and pinealocyte cultures express circadian patterns of AANAT activity (Binkley et al. 1977a; Kasal et al. 1979; Wainwright and Wainwright 1979) and melatonin efflux (Takahashi et al. 1980) such that levels are high during the night and low during the day in LD. These rhythms persist for 4–10 days in DD before damping to arrhythmicity. Exposure to light has three effects on cultured pineal rhythms: (1) Light inhibits melatonin biosynthesis. (2) Light increases amplitude and decreases damping, and (3) light phase-shifts the clock within pineal cells (Zatz and Mullen 1988a). In vivo, the avian pineal gland is innervated by post-ganglionic sympathetic nerves, and receives daily and circadian input through release of norepinephrine (NE) during the day and subjective day. Administration of NE to chick pineal glands in vivo and in vitro has two effects on pineal melatonin rhythms: (1) NE inhibits melatonin biosynthesis. (2) NE increases amplitude and decreases damping, but does not phase-shift the pineal circadian clock (Cassone and Menaker 1983; Cassone et al. 1986; Zatz and Mullen 1988b).
Using cDNA microarrays of chick pineal gland in vivo and in vitro and retinae in vivo, we have shown that many clock genes are expressed rhythmically in a fashion consistent with circadian patterns of clock gene expression in other model systems, such as Drosophila and mice (Bailey et al. 2003, 2004; Karaganis et al. 2008). In vivo, bmal1, bmal2 and clock are expressed predominantly during late subjective day/early subjective night, while putative negative elements per2 and per3 (per1 is not present avian genomes) are expressed during the subjective night to early subjective day. Interestingly, cry1 and cry2 are expressed during the early to mid-subjective day. In addition, transcripts associated with melatonin biosynthesis and photoreception are expressed rhythmically in patterns that are consistent with previous studies described above. Importantly, the 5′-flanking region of the chicken aanat gene contains an E-box (Chong et al. 2000), indicating the CLOCK-BMAL1 dimer may regulate aanat expression in the late subjective day/early subjective night. Transfection of COS cells with a reporter construct expressing luciferase and the chicken aanat promoter region in the presence of either chicken or human BMAL1 and CLOCK increases luciferase activity (Chong et al. 2000). Mutation of the E-box region dramatically decreases luciferase activity, suggesting that binding of the aanat E-box by CLOCK/BMAL1 heterodimers is a critical component of the regulation of melatonin biosynthesis (Chong et al. 2000; Kasal et al. 1979) Interestingly, in pineal glands, but not retinae, many transcripts associated with cytokine biosynthesis, immune function, and lymphopoiesis are both highly and rhythmically expressed in chick pineal gland. These data are similar to those obtained by in situ hybridization in Japanese quail (Yasuo and Yoshimura 2009; Yasuo et al. 2004).
Although in situ hybridization for clock genes suggest that these genes are enriched in the pineal gland, retinae, and other structures associated with clock function (Bailey et al. 2003, 2004; Wiltschko and Wiltschko 2013), quantitative real-time PCR for these transcripts indicate they are widely expressed in other parts of the brain and in the periphery. Daily and circadian patterns of cry1, per3 and bmal1 expression were observed in chick telencephalon, diencephalon, and optic tectum in the brain, as well as in heart and liver. PINX and EX of chicks decreases the amplitude of these clock gene rhythms but does not completely abolish them (Karaganis et al. 2009).
Interestingly, the photoreceptors in the retinae of the lateral eyes also synthesize and release melatonin in many vertebrate species. In fact, in Japanese quail and domestic pigeon, Columba livia, the retinae release almost as much melatonin into the systemic circulation as does the pineal gland and removal of this source by enucleation (EX) or retinectomy in addition to PINX results in arrhythmic circadian locomotor behavior, similar to the effects of PINX alone in passerine birds (Chabot and Menaker 1992; Ebihara et al. 1984; Underwood and Siopes 1984). Thus, the variability of the effects of PINX among birds may in part be due to this retinal component in some species and that it is not the pineal per se but rhythmic melatonin that is important for circadian locomotor behavior. To punctuate this view, rhythmic administration of melatonin to PINX house sparrows and European starlings or to EX/PINX pigeons (Chabot and Menaker 1992; Gwinner et al. 1997; Heigl and Gwinner 1995; Lu and Cassone 1993; Wang et al. 2012) restores a daily pattern of locomotor behavior. This synchronization of locomotor behavior by rhythmic melatonin administration represents entrainment of circadian clockworks in the PINX bird, since melatonin administration in a T-cycle different from 24 h results in systematic changes in the phase relationship (ψ) of melatonin to the onset of locomotor activity (Gwinner et al. 1997; Heigl and Gwinner 1995).
5.6 Sites of Melatonin Action in Birds
In the 1980s and 1990s, high affinity melatonin receptor binding using the radiolabeled agonist 2[125I]-iodomelatonin (IMEL) (Vakkuri et al. 1984) revealed high densities of IMEL binding in retinal, retinorecipient structures, and visual integrative structures in the avian brain as well as peripheral tissues (Dubocovich and Takahashi 1987; Rivkees et al. 1989) (Fig. 5.3). Binding affinity studies indicated kDs in the pM range with high specificity for melatonin itself. Brain structures that bind IMEL included retinorecipient structures in the visual suprachiasmatic nucleus (vSCN) of the circadian system, the ventrolateral and dorsal geniculate nuclei of the thalamofugal visual pathway, the optic tectum of the tectofugal pathway, and the nucleus of the basal optic root (nBOR) or the accessory optic pathway. In all species, integrative structures of the tectofugal pathway such as nucleus rotundus (Rt) and the ectopallium (Ep) also bind IMEL (Cassone et al. 1995; Rivkees et al. 1989). In some but not all species, hyperpallial structures, including the visual Wulst are sites of IMEL binding. In male passerine birds but not females, structures associated with bird song learning and control also revealed high affinity IMEL binding (Gahr and Kosar 1996; Whitfield-Rucker and Cassone 1996). These will be discussed in more detail below.
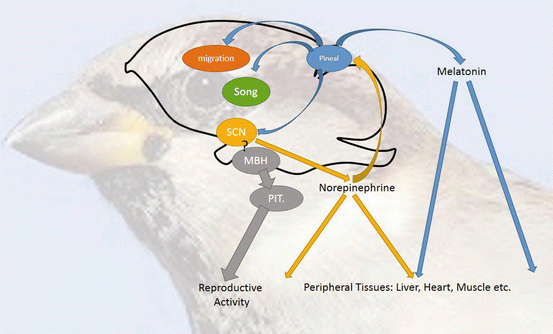

Fig. 5.3
A chorus of clocks. The coordination of circadian and circannual patterns of behavior and physiology is the composite of multiple circadian oscillators and their interactions. At the core are circadian pacemakers in the pineal gland and suprachiasmatic nuclei (SCN), whose interactions maintain each other’s stability and self-sustainment through mutually inhibitory activity. Each of these pacemakers may influence downstream processes through entrainment of circadian oscillators controlling and residing in brain (e.g., song, vision, and migration) and peripheral (e.g., liver, heart, and muscle) function. In birds, regulation of primary gonadal activity has been separated from this circadian system with circadian oscillators residing in the mediobasal hypothalamus itself. There is little evidence that these oscillators are affected by pineal melatonin, but it is an open question whether SCM oscillators influence MBH function in the circadian and seasonal control of reproduction
Reppert and colleagues were able to isolate and clone genes encoding two high affinity melatonin receptors; these were designated the Mel1A and Mel1C receptors (Reppert et al. 1995). Independent work isolated partial sequences encoding an ortholog of the Mel1B receptor in the same year (Liu et al. 1995). Subsequent work has confirmed in birds that there are at least three melatonin receptors, the Mel1A, Mel1B, and the Mel1C receptors (Reppert 1997). Pharmacologists working with mammals have named the Mel1A and Mel1B receptors MT1 and MT2 receptors, respectively (Dubocovich et al. 2010). However, mammals do not express the Mel1C receptor sub-type, and the Mel1A and Mel1B receptors have not been fully characterized pharmacologically in birds. In this review, we will employ the original nomenclature, the Mel1A, Mel1B, and the Mel1C receptors. All three melatonin receptor subtypes represent seven-transmembrane domain, GTP-binding protein structures and all three are in the Gi GTP-binding protein category, although some cross-talk with Gq has been documented (Reppert 1997). The distributions of these three receptor subtypes are not uniform in chicks, zebra finches, and house sparrows. The Mel1A receptor predominates in central nervous neurons and peripheral tissues (Karaganis et al. 2009; Natesan and Cassone 2002), while the Mel1B receptor is expressed in inner retinal neurons and photoreceptors as well as other central nervous neurons (Natesan and Cassone 2002). In passerines, the Mel1B receptor is the major receptor subtype in song control nuclei but the other two are expressed as well (Bentley et al. 2012; Jansen et al. 2005). The Mel1C receptor, on the other hand, predominates in non-neuronal elements of the central nervous system (Reppert et al. 1995). Culture studies with chick astrocytes (Adachi et al. 2002) show 95–100 % diencephalic astrocytes express the Mel1C receptor, while an overlapping 5–10 % expresses Mel1A. Astrocytes do not appear to express Mel1B. Intriguingly, neither IMEL binding nor strong melatonin receptor expression is present in the tuberal hypothalamus and/or hypophysis (Cassone et al. 1995; Reppert et al. 1995, Rivkees et al. 1989).
5.7 Avian Circadian Organization
Elimination of the pineal gland’s photic and neural inputs results in damped circadian patterns of melatonin release, which also can be restored by rhythmic administration of light and/or NE (Cassone and Menaker 1983; Takahashi et al. 1980; Zatz and Mullen 1988a, b). Conversely, removal of the pineal pacemaker in passerine birds at least results in damped rhythmicity in behavioral and physiological output, suggesting whatever is left represents a damped circadian oscillator capable of entraining to light (Gaston and Menaker 1968). Whatever is left is likely at least in part the avian homolog for the mammalian hypothalamic nucleus (SCN) (Cantwell and Cassone 2006a, b), which is a master pacemaker for mammalian circadian organization (cf. Moore and Silver 1998).
In birds, two sets of structures have been associated with SCN function: the medial suprachiasmatic nuclei (mSCN) and the visual suprachiasmatic nuclei (vSCN) (Cantwell and Cassone 2006a, b; Cassone and Moore 1987; Yoshimura et al. 2001). These structures are connected via neuronal projections and are contiguous in terms of their cellular populations, especially in the distribution of astrocytes. The vSCN, but not the mSCN, expresses metabolic and electrical rhythmicity and receives retinohypothalamic (RHT) input. Further, the vSCN, but not the mSCN, contains melatonin receptor binding (Cassone et al. 1995; Lu and Cassone 1993; Reppert et al. 1995; Rivkees et al. 1989), and exogenous melatonin inhibits metabolic activity in the vSCN (Lu and Cassone 1993) but not in the mSCN. Finally, light activates c-fos expression in the vSCN, but not in the mSCN (King and Follett 1997). In quail, only the mSCN expresses clock gene rhythmicity (Yasuo and Yoshimura 2009; Yasuo et al. 2002), while in the house sparrow, both structures rhythmically express per2 rhythms (Abraham et al. 2002). Lesions directed at the mSCN result in arrhythmicity similar to that observed following PINX (Takahashi and Menaker 1982). The most likely scenario is that the functions subsumed by the mammalian SCN are regulated through the integration of both mSCN and vSCN.
Each component, the pineal gland, retinae, and SCN, is integrated dynamically such that overt circadian organization is synchronized to environmental light cycles (LD) and such that internal processes are adaptively orchestrated (Cassone and Menaker 1984; Gwinner and Brandstätter 2001). Pineal (and retinal) melatonin, synchronized to LD cycles via endogenous photopigments, is secreted during the night and inhibits rhythmic metabolism and electrical activity of the vSCN. In turn, as the day approaches, oscillators within the pineal gland and retinae wane in their output, disinhibiting SCN activity. Oscillators within the mSCN and vSCN are active during the day, synchronized by LD via RHT input to the vSCN and possibly extraretinal input to the mSCN. One of the outputs of the vSCN at least is the rhythmic regulation of sympathetic activity, releasing norepinephrine (NE) within many peripheral targets. Among these is the pineal gland, where NE inhibits melatonin biosynthesis and release. It is not completely clear whether sympathetic NE synchronize circadian clockworks within the pineal gland, nor is it clear whether pineal melatonin affects clock gene expression in the SCN in vivo. However, in vitro, rhythmic melatonin administration synchronizes rhythms of both metabolic activity and the expression of both per2 and per3 (Paulose et al. 2009).
5.8 Circadian Regulation of Visual System Function
The presence of dense, high affinity melatonin receptors within the retina, retinorecipient structures of all four visual pathways, and visual integrative structures in the brains of multiple avian species (Cassone et al. 1995), strongly suggests visual sensitivity, accommodation, and more complex aspects of visual perception may be regulated on a circadian basis by melatonin. Circadian patterns in electroretinogram (ERG) and visually evoked potentials (VEP) recorded within the TeO show greater response amplitude during the day in LD and during subjective day in DD in both domestic chicks and pigeons (Lu et al. 1995; Peters and Cassone 2005; Wu et al. 2000). In chicks and pigeons, the implicit time of the A-wave, which reflects photoreceptor activity, and B-wave, which reflects inner retinal activation, are greater during the night and subjective night than during the subjective day, while light sensitivity is greatest during the subjective night, reflecting the activity of dark-sensitive rods (Peters and Cassone 2005; Wu et al. 2000). PINX of chicks reduces the amplitude of the circadian rhythm in ERG amplitude (McGoogan and Cassone 1999), and exogenous melatonin injections decreases ERG b-wave during the subjective day to levels similar to those described at night (McGoogan and Cassone 1999); (Peters and Cassone 2005). At this stage, it is not clear whether the effects of melatonin on avian visual system function directly influence physiological processes within visual system structures and/or regulate circadian clocks within the brain. The avian TeO expresses rhythms in clock gene expression in areas (Yasuo et al. 2004) superimposed with high affinity melatonin receptors (Cassone et al. 1995), but the physiological link has yet to be made. Further, it is not clear how higher order visual function is affected by the circadian clock and melatonin.
Interestingly, this pattern of circadian regulation of visual system function, at least at the ERG level is nearly identical in humans with high implicit times during the night and high b-wave amplitudes during the day (Hankins et al. 1998; Nozaki et al. 1983). Further, administration of exogenous melatonin to human volunteers decreases b-wave amplitude during the day (Gagné et al. 2009), similar to the situation in birds (McGoogan and Cassone 1999; Peters and Cassone 2005). The role of melatonin in retinal physiology and pathophysiology is an emerging area of research (Tosini et al. 2012), and birds provide an excellent model for human visual physiology in this regard.
5.9 Seasonal Cycles and Photoperiodism in Birds
Birds living in temperate zone latitudes generally restrict breeding to the spring and summer, maximizing the likelihood that young will be hatched during times at which food is plentiful (Gwinner 1989; Gwinner et al. 1997; Pittendrigh 1993). As such, many primary and secondary sexual characteristics in birds undergo dramatic changes in both form and function. In the short days of winter, gonadal activity and gonad size regress and become inactive, while gonads recrudesce, becoming more active, in response to increased photoperiod. If birds are maintained in long photoperiod, their reproductive systems become insensitive to the photostimulatory effects of long photoperiod and spontaneous regress. This process is called photorefractoriness, and birds remain photorefractory until they are placed in short days for some time to make them photosensitive again (Balthazart et al. 2010; Goldsmith et al. 1989; Kumar et al. 1996; Pittendrigh 1993).
In seasonally reproducing mammals such as hamsters and deer-mice, the nocturnal secretion of pineal melatonin is a critical signal that transduces time of year to the hypothalamo–hypophysial–gonadal axis to control reproductive function (Bartness et al. 1993; Goldman 2001). The duration of pineal melatonin biosynthesis faithfully reflects the duration of the scotoperiod; the duration is long during the long nights of winter and short during summer. These animals also exhibit a seasonal cycle of reproductive activity in which testes in males and estrus cyclicity in females increase as photoperiod increases in the spring. When photoperiod decreases, testes regress and females become anestrus. PINX in several species of hamsters prevents regression of gonads when the hamsters are transferred from long photoperiods to short photoperiods; their reproductive systems become blind to photoperiodic changes. When melatonin is infused into PINX Djungarian hamsters, Phodopus sungorus, long durations of melatonin, simulating winter, induce gonadal regression, while short durations, simulating summer, enable recrudescence. The sites for melatonin’s activity in this process are a combination of melatonin receptors in the SCN and in the pars tuberalis of the hypophysis. Therefore, the circadian control of pineal melatonin regulates the annual control of reproduction. This brief summary of mammalian seasonality is necessarily short in a review about birds; extensive reviews of these mechanisms can be found elsewhere (cf. Goldman 2001; Hastings and Follett 2001).
Intriguingly, in spite of the fact that the rhythmic production of melatonin is critical for the expression of circadian locomotor rhythms in birds, melatonin does not affect seasonal changes in primary reproductive function in these species. As in mammals, pineal melatonin levels faithfully reflect the length of the scotoperiod both in vivo and in vitro (Binkley et al. 1977b; Brandstätter et al. 2000). However, PINX and/or EX of several species of birds has little effect on seasonal changes in gonad size or activity (Bentley 2001; Kumar et al. 2002; Schleussner and Gwinner 1989; Sharp 2005; Siopes 1983). Moreover, administration of exogenous melatonin of different durations has little effect on primary reproductive function (Cassone et al. 2008; Meddle and Follett 1997). This corresponds to the relative absence of melatonin receptor activity in the tuberal hypothalamus and hypophysis (Cassone et al. 1995), in stark contrast to the situation in seasonally reproducing mammals, where IMEL binding and melatonin receptor expression in pars tuberalis is a major site of melatonin action (Goldman 2001; Hastings and Follett 2001).
Yoshimura and colleagues have instead pointed to circadian clock function within the mediobasal hypothalamus (MBH) itself of Japanese quail controlling photoperiodic time measurement for reproductive function (Yoshimura 2010). Earlier studies had shown that lesion of the MBH blocked testicular recrudescence in response to lengthening photoperiods and illumination of this area had resulted in excitation of the tuberal hypothalamus and testicular growth (Foster et al. 1985; Kumar et al. 2010; Nakao et al. 2007). As stated above, the MBH of quail and the PMM of turkeys have been shown to express both OPN4 and OPN5 in cerebrospinal fluid (CSF) contacting neurons (Kang et al. 2010; Nakane et al. 2010; Nakao et al. 2007). Noting that PINX or EX or even SCN lesion had little effect on photoperiodic regulation of gonadal function, Yoshimura’s group identified rhythmic expression of the clock genes in the MBH and hypothesized that this structure contained the circadian pacemaker associated with photoperiodic time measurement (Nakao et al. 2008a; Ono et al. 2009). Using differential subtractive hybridization, they found type 2 iodothyronine deiodinase (Dio2) to be induced in the MBH by a light pulse associated with long-day induction. Dio2 encodes an enzyme that catalyzes the conversion of inactive thyroxine (T4) to active triiodothyroine (T3) (Nakao et al. 2008a, b; Yasuo and Yoshimura 2009; Yasuo et al. 2006). Later, they showed that type 3 iodothyronine deiodinase (Dio3), which inactivates T3, was induced in the MBH by exposure to short days. The scenario they envision is that photoperiod is perceived by photopigments in the MBH that entrain a circadian oscillator within the MBH. In long days the circadian clock induces Dio2, while short days induce Dio3. Indeed, thyroidectomy induces gonadal recrudescence in starlings and Japanese quail (Dawson et al. 1986; Follett and Nicholls 1985) and injection of T3 into the MBH induces quail gonadal growth (Follett and Nicholls 1985; Yoshimura et al. 2003). These authors envision an external coincidence model in which circadian oscillators within the MBH are entrained by photoperiod colocalized in that structure. Gonadal recrudescence in starlings a ϕ pi, Dio2 is induced, enabling a metabolic cascade in response to T3 hormone, and gonadal induction occurs. It is not clear, at this stage, what molecular components link the circadian clock to Dio2 or Dio3. The MBH of quail and the PMM of turkeys rhythmically express clock genes (Ikegami and Yoshimura 2012; Ikegami et al. 2009; Leclerc et al. 2010). In humans, Dio2 is regulated by CCAAT/enhancer-binding proteins, and per2 is known to be a target of these transcription factors as well (Gegear et al. 2010; Thoennissen et al. 2012). Perhaps, this link may provide clues to an analogous mechanism in birds.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


