CHAPTER 63 Assisted Reproductive Technologies in Cattle
Assisted reproductive technologies (ART) have made tremendous advances, especially during the past 15 years. Artificial insemination (AI) remains the most effective method for achieving genetic progress in populations of cattle. The global market remains strong for frozen semen and embryos. Millions of cattle are bred by AI, and more than a half million embryos are transferred annually world wide. Most of the top dairy sires used for AI were derived from embryo transfer (ET). Improvements in methods of controlling the estrous cycle and ovulation have resulted in more effective programs for AI, superovulation of donor cows, and the management of ET recipients. The recent introductions of in vitro embryo production, cloning, and sexed semen have added to the ART “toolbox.”
EMBRYO TRANSFER
The next major commercial advancement in reproductive biotechnology was embryo transfer in the late 1970s. ET enabled the acceleration of the proliferation of genetic material from the dam as well as of the sire. The ability to freeze and transport bovine embryos around the world has made ET an extremely useful technology for disease control and biosecurity programs, genetic salvage of valuable individuals, and development of new lines or breeds of cattle. ET is a factorial process that depends on a series of carefully orchestrated sequential steps. Poor performance in any of the steps directly affects the success rate and the final outcome, the number of calves weaned.
Donor Management
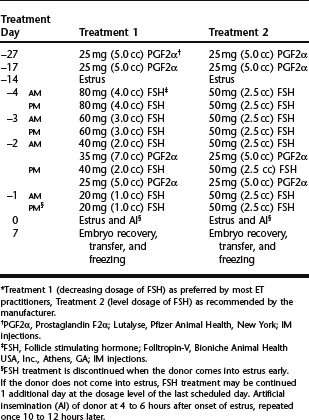
Superovulation remains the least predictable step in the process of embryo production. In the bovine tremendous variation in response occurs with age, breed, lactational status, nutritional status, season, and stage of the cycle at which treatment is initiated. Follicle stimulating hormone (FSH), which has a short half-life, necessitates twice-daily injections over a period of 4 to 5 days. Treatment is begun during the mid-luteal phase (day 8 to 12) of the donor’s cycle and employs the use of prostaglandins (PG) to synchronize the cycles of the donors and the recipients. Alternatively, treatment may be initiated on day 16 or 17 (day 0 = estrus) of the donor’s natural estrous cycle. Currently the most commonly used source of FSH in the United States is Folltropin-V.* Twice daily intramuscular injections of FSH are recommended (Table 63-1). Prostaglandins (25–35 mg PGF2α or 500 μg PG-analogue IM) are routinely given at the time of the fifth and sixth FSH injections of a 4-day regimen, which is then followed by estrus in 2 days and ovulation in 3 days. This interval from PG to the onset of estrus is 12 to 24 hours shorter in superovulated animals than in naturally ovulating cows or heifers. Consequently, recipients should be injected with PG 24 hours before the donors if this method of synchronization is used. The response to the FSH regimen ranges from zero to 20 or more ovulations with an average of 8 to 10. There appears to be no difference in response between a 4-day and a 5-day regimen. Generally, heifers require a smaller total dose and older animals a higher total dose.
Many embryo transfer practitioners use exogenous progesterone or intravaginal controlled drug (progesterone) release devices (controlled internal drug release, CIDR)† as part of the superovulation protocol. This approach may be used simply to synchronize a group of donors in order to start the FSH treatment approximately 10 days after their synchronized heat. Alternatively, the donors may be superovulated with the CIDR in place according to the treatment schedule in Table 63-2. The advantage of the latter approach is that the donors may be implanted at any time during their cycle. However, it is critical that the donor is a reproductively normal, cycling animal.
Table 63-2 Superovulation Treatment with Follicle Stimulating Hormone and CIDR without Regard to the Day of the Donor’s Estrous Cycle
| Treatment Day | Treatment | |
|---|---|---|
| 0 | CIDR* inserted vaginally | |
| 2 | PM | 100 μg GnRH† |
| 4 | PM | 60 mg (3.0 cc) FSH‡ |
| 5 | AM | 60 mg (3.0 cc) FSH |
| PM | 60 mg (3.0 cc) FSH | |
| 6 | AM | 50 mg (2.5 cc) FSH |
| PM | 50 mg (2.5 cc) FSH | |
| 7 | AM | 40 mg (2.0 cc) FSH |
| PM | 40 mg (2.0 cc) FSH, 35 mg (7.0 cc) PGF2α§ | |
| 8 | AM | 40 mg (2.0 cc) FSH, 25 mg (5.0 cc) PGF2α |
| CIDR out | ||
| 9 | AM | Estrus and Al |
| PM | Estrus and Al | |
| 16 | Embryo recovery, transfer, and freezing | |
* CIDR, Controlled internal drug release; EAZI-BREED CIDR progesterone insert, InterAg Company, Hamilton, NZ, or Pfizer Animal Health, New York.
† GnRH, Gonadotropin releasing hormone; Cystorelin (Gonadorelin), Merial Limited, Iselin, NJ; IM injection.
‡ FSH, Follicle stimulating hormone; Folltropin-V, Bioniche Animal Health USA, Inc., Athens, GA; IM injections.
§ PGF2α, Prostaglandin F2α; Lutalyse, Pfizer Animal Health, New York; IM injections.
Embryo Recovery
The donor is restrained in a chute or in stocks. Nervous animals may be given 5 to 10 mg of acepromazine or another suitable tranquilizer. Feces are carefully removed from the rectum to avoid aspiration of air, and an estimate is made of the number of ovulations (CL). Epidural anesthesia is administered (4 to 6 ml of 2% lidocaine hydrochloride) to prevent defecation and straining. Fractious animals may be given epidural anesthesia with a combination of xylazine (30 mg) and sterile saline or sterile water (7 ml or sufficient quantity). Bos indicus breeds are more sensitive to the action of xylazine and should receive 20 mg of xylazine in a sufficient quantity, usually 7 ml, of sterile saline. Inadvertent air can be removed from the rectum with a small stomach tube attached to a wet vacuum cleaner. The vulva and perineal region are thoroughly washed with plain water and blotted dry. The tail is tied out of the way. If the cervix feels small or tortuous, a cervical dilator may gently be used to expand and straighten the cervical canal. The dilator and subsequent catheters may be covered with a sanitary sleeve before they are introduced into the vagina. This protective cover is perforated just before the instrument enters the external os of the cervix. The rigid, relatively sharp-pointed dilator should be used with extreme caution as it can readily perforate the uterine wall when it is forced through the tight cervical canal. The lips of the vulva are again parted and the balloon catheter, with the stylet in place, is inserted into the vagina and advanced into the lumen of the cervix. It is then manipulated into the appropriate horn until the inflatable balloon is situated at the base of the uterine horn. The balloon is slowly inflated with 15 to 20 ml of air or flushing medium in adult cows and 10 to 15 ml of air in heifers. The endometrium can easily be split by overdistention, resulting in hemorrhage and escape of the flushing solution into the mesometrium, from which it cannot be recovered.
Flushing and Holding Media
By the very nature of the procedure, it is vital that all aspects of quality control of media and equipment that come in contact with the embryos are strictly adhered to. It is also advisable to use commercially prepared media and sterile disposable supplies.1
Environment
Exposing the embryos to ultraviolet rays for a prolonged period may cause cellular death. The use of insecticide sprays in the embryology room should be avoided. Insufficient time of aeration after using ethylene oxide gas for sterilization of equipment is detrimental to live cells. Storage period, different suppliers, and batches (lot number) of sera all affect embryo growth differently.
Handling the Embryos
Once an embryo is identified in the searching dish, it is immediately transferred to a small Petri dish (35 × 10 mm) containing fresh, filtered (0.22 μm pore size), sterile holding medium. Embryos are tentatively classified simply as good or bad, and may be recorded on the cover of the holding dish. This allows for a quick account of the total number of embryos found. Embryos are then serially rinsed through at least three different dishes containing fresh sterile medium using a new sterile pipet for each step. Finally, they are placed into a dish awaiting transfer or cryopreservation. Under some circumstances (e.g. for export of embryos) they must be rinsed through 10 different dishes containing sterile media and exposed to trypsin.1 All dishes must be kept covered between searches to avoid contamination, and particularly evaporation, when placed in the incubator. Evaporation of the small volume of medium in a flat dish rapidly leads to hypertonic solutions.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree