CHAPTER 56 Aspergillosis
Aspergillus species are among the most common fungal organisms. These saprophytes are widely distributed in the environment in soil, decaying vegetation, and organic debris. The members of this genus are opportunistic invaders of animal tissues, and healthy animals are resistant to infection unless exposed to a massive number of conidia or mycelia. Risk of disease caused by Aspergillus spp. is thought to be increased in immunodeficient horses and in horses with concurrent, severe enterocolitis or hematopoietic neoplasia. Transmission is usually by aerosol; guttural pouch mycosis and pulmonary disease are recognized manifestations of Aspergillus infection in horses. Pulmonary lesions are characteristically granulomatous. Keratomycosis caused by Aspergillus spp. is also common in horses.
ETIOLOGY
The classification and identification of Aspergillus species are based primarily on morphologic characteristics of the organism in culture. The genus Aspergillus is in the family Trichocomaceae of the order Eurotiales in the class Plectinomycetes of the phylum Ascomycota.1 Fungi of the phylum Ascomycota are characterized by telomorphic (sexual) reproduction. Some textbooks2 classify Aspergillus in the phylum Deuteromycota (fungi imperfecta), probably because this phylum contains anamorphic organisms, for which sexual reproduction has not been identified. The sexual state is found in very few species of Aspergillus, and the anamorphic (asexual) state is usually encountered in cultures from clinical patients.
Morphologic characteristics of Aspergillus spp. vary in culture. In general, Aspergillus spp. spread rapidly over the medium, forming a mycelium of flat to aerially branching and interlacing hyphae and conidiophores. The mycelium may be powdery white, greenish yellow, brown, or black.1 The hyphae are nonpigmented and septate. Conidiophores (stalks) are characterized by a vesicle at the tip. The vesicle bears papillae (phialides) that give rise to unicellular conidia (cells produced by asexual reproduction) that are arranged in chains (Fig. 56-1). The characteristic shape of the conidiophore and spore heads is responsible for the name Aspergillus, which was conferred on the organism by an eighteenth-century priest who noted the resemblance of this pattern to that of a holy water sprinkler (“Aspergillum”).1 Conidia may be colorless or darkly pigmented.
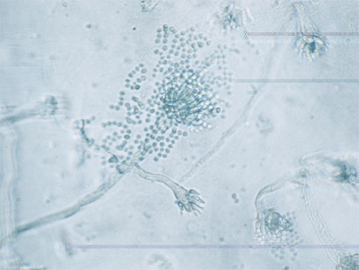
Fig. 56-1 Photomicrograph of Aspergillus conidiospores.
(Courtesy Dr. Joseph Kowalski, The Ohio State University College of Veterinary Medicine.)
Approximately 180 species of Aspergillus are currently recognized, of which about 40 species have been identified in opportunistic infections of humans. Clinical studies of aspergillosis in horses usually report the presence of Aspergillus spp., and many do not identify the particular species involved. Aspergillus fumigatus is the most common species identified. Aspergillus niger3,4 and Aspergillus versicolor5 have also been reported to cause disease in horses. One case of keratomycosis caused by Aspergillus oryzae has been reported.6 Mycotoxins produced by Aspergillus flavus cause equine aflatoxicosis.7
Virulence Factors
Because of their small size (2-3 μm in diameter), conidia of Aspergillus spp. remain in suspension in air for a long time, and these respirable particles can efficiently penetrate to the alveoli.8 Aspergillus spp. are ubiquitous. The environment of the horse and particularly the “breathing zone” are almost continually rich in hyphae and conidia of Aspergillus, and people and horses constantly inhale fungal elements. In healthy horses and persons, fungi are cleared from the respiratory tract by pulmonary defense mechanisms (mucociliary clearance, phagocytosis by pulmonary macrophages). Aspergillus spp. are thermotolerant. The organisms thrive at 37° C (98.6° F), are able to grow at 55° C (131° F), and are reported to survive at temperatures up to 75° C (167° F).9 These fungi are thus able to grow rapidly in body tissues of immunoincompetent hosts.
Aspergillus spp. survive in the bloodstream and tissues by being able to find and assimilate essential growth nutrients in these alien environments. The ability of these organisms to sense nitrogen sources in the environment and to regulate nitrogen utilization is essential to their survival.10 Other fungal attributes that support the acquisition of essential nutrients include siderophores, phosphatases, and phospholipases. Fungal organisms require iron for growth, and blood is “fungistatic” because most iron is bound to transferrin.9 Aspergillus fumigatus produces at least six siderophores that are able to remove iron from transferrin in vitro.8,9 A. fumigatus has a high phosphate requirement. Phospholipases and phophatases allow the organism to recover phosphate from environments that are not phosphate rich, such as blood, and to utilize inorganic phosphates.
Aspergillus spp. may act as allergens. Allergic bronchopulmonary aspergillosis, allergic rhinosinusitis, asthma, and aspergilloma have been reported in immunocompetent human patients.8 Inhalation challenge with extracts of A. fumigatus in horses with recurrent airway obstruction can induce signs of this disease.11
Gliatoxin and other toxins are putative virulence factors produced by A. fumigatus.12 These factors may have direct toxic effects on tissues, and many are immunosuppressive.
A number of fungal characteristics assist Aspergillus spp. in evading host immune responses. Melanin pigment on the surface of conidia can neutralize reactive oxygen species and protect the organisms against attack by macrophages and neutrophils.9 Aspergillus spp. have extensive antioxidant capabilities and many efflux pumps to facilitate export of toxins. Hydrophobins form part of the external layer of the cell wall of the conidia and may protect them from destruction by alveolar macrophages.9 In vitro, conidia and hyphae may be phagocytosed by endothelial cells and tracheal or alveolar epithelial cells.8 In theory, fungi sequestered in these cells could evade immune surveillance. Some investigators speculate that Aspergillus organisms may be able to “direct” their phagocytosis by these “nonprofessional” phagocytic cells during times of environmental stress.8 This ability to invade endothelial and epithelial cells may be a mechanism that facilitates angioinvasion and thrombosis by Aspergillus spp. in vivo.8
EPIDEMIOLOGY
Aspergillus spp. are ubiquitous in the environment of horses, as previously discussed. In one study, 67.8% of the fungi recovered from air and surface samples taken in three stables in the winter were Aspergillus spp.13 Fungal contamination can be reduced by management changes; more environmental fungi were recovered from stables with wooden stalls, dirt floors, and straw bedding than from hospital stalls with masonry walls, synthetic flooring, and bedding composed of wood shavings.14 Not surprisingly, Aspergillus spp. may be encountered in the conjunctiva 15–18,20 (50% of 43 horses sampled in one study19,20) and in airway fluid samples obtained by transtracheal aspiration of healthy horses.21 Conidia may be found free in the transtracheal wash fluid or within large mononuclear cells. Aspergillus can also be routinely cultured from the ingesta,22 the skin, and the caudal reproductive tract, including the external genitalia.23
Mycotic Keratitis (Keratomycosis)
Aspergillus spp. are the most common cause of keratomycosis in horses. Case review studies have shown that this organism can be isolated from 33% to 77% of horses with keratomycosis24–27 and 2% to 22% of eyes from horses with external eye disease.17 Fusarium spp. are also encountered frequently (10 of 39 horses [26%] with keratomycosis in one study28), whereas Alternaria, Cladosporium, Pseudallescheria, Geotrichum, Scedosporium spp., Stemphylium spp., Penicillium, Cylindrocarpon, Scytalidium, and Torulopsis are infrequently reported.25,28 Nonseptate filamentous fungi such as Mucor and yeasts such as Candida may also be associated with mycotic keratitis. The mycology of keratomycosis may vary with the geographic region. Mycotic keratitis is thought to be rare in the United Kingdom, although a recent report documented six cases (1998–2002).29
Although mycotic keratosis may occur more frequently during the summer and fall in some regions, cases are seen year-round in Florida, where the majority of cases are reported to occur October through January.28 No apparent age, breed, or gender predisposition exists for mycotic keratitis.
Endometritis and Placentitis
The most common causes of mycotic endometritis are Aspergillus spp. and Candida spp.23,30 Mycotic infections account for 1% to 5% of cases of endometritis in mares.
Aflatoxicosis
Aspergillus flavus and A. parasiticus produce toxic metabolites known as aflatoxins. Corn, peanuts, peanut meal, and cottonseed meal are the most frequently contaminated sources of aflatoxins. The toxins are also found in other nuts (pecans, walnuts, almonds, hazelnuts).7 Ambient temperatures between 78° and 90° F (25.5°-32° C), drought stress, and high relative humidity or grain moisture favor production of aflatoxins.31 Factors that reduce protection of the seed or damage the seed coat, such as shortened husks or insect damage, also enhance the risk of aflatoxin production.
PATHOGENESIS
The virulence of Aspergillus organisms is usually a function of the immune incompetence of the host, rather than the presence of specific virulence factors intrinsic to the fungus.9 Because this fungus is ubiquitous, it is frequently inhaled, ingested, or contacted directly (e.g., corneal surface) by potential hosts. Those hosts with weakened innate or acquired immune responses are at risk for colonization by this opportunistic organism. Invasive aspergillosis has become an important disease in patients with acquired immunodeficiency syndrome (AIDS) and in other human patients who are severely granulocytopenic because of their disease or immunosuppressive treatment with anticancer drugs or corticosteroids. Invasive aspergillosis has been reported to occur in 70% of patients who remain granulocytopenic for 34 or more days.32 A CD4 lymphocyte count less than 50 cells/mm3 is a risk factor for invasive aspergillosis in AIDS patients.32 Leukopenia and neutropenia are often associated with enterocolitis and typhlitis in horses.
In horses, immune incompetence is assumed to be an important risk factor for invasive aspergillosis, an otherwise uncommon disease. Pulmonary aspergillosis has been reported in a horse treated for ill thrift and intestinal malabsorption with corticosteroids22 and in another with dysfunction of the pars intermedia of the pituitary.33 Hyperadrenocorticism in horses with hyperplasia of the pars intermedia may be immune suppressive.34 Invasive aspergillosis has been reported in four horses with hemolymphatic neoplasia (three with myelomonocytic leukemia and one with disseminated hemangiosarcoma).22,35,36 Several investigators have noted an association between invasive aspergillosis and antecedent enterocolitis.4,22,34,37–39 In one study, pulmonary aspergillosis developed in a mean of 11 days after enterocolitis in 16 horses (14 with salmonellosis).22 In another study, 25 of 29 horses with pulmonary aspergillosis had evidence of primary or secondary disease that caused loss of the integrity of the bowel wall; 12 of these horses were leukopenic.34 Loss of integrity of the bowel mucosa likely provided a portal for absorption of fungi from the bowel lumen, allowing embolic spread of the organisms to other organs, particularly the lungs, but also kidney and brain.4,40 Broad-spectrum antibiotic therapy may contribute to the problem by destroying symbiotic bacteria that balance the gastrointestinal flora, preventing growth of potential pathogens.
Aspergillosis is occasionally reported in immunocompetent horses.3,41,42 Fatal pneumonia caused by Aspergillus niger and Rhizopus stolonifer was diagnosed in two young horses recently moved to an unused, unclean stable.3 The organisms were recovered from the lungs of affected horses and from the stable bedding. A third case of fatal invasive pulmonary aspergillosis was reported in an immunocompetent horse housed in a bank barn.41 These authors hypothesize that affected horses were exposed to overwhelmingly large aerosol doses of fungi. Aspergillus spp. were isolated from a large, well-encapsulated mediastinal mass at the base of the heart in an otherwise healthy horse.42
Antigens of Aspergillus fumigatus have been implicated in the pathogenesis of recurrent airway obstruction (RAO) in horses. Inhalation challenge with an extract of A. fumigatus induced increased pulmonary resistance in horses with RAO43 and caused signs of airway obstruction in RAO-affected horses in remission, but not in healthy control horses.11 Serum allergen-specific immunoglobulin E (IgE) concentrations suggest that horses with RAO are more often sensitized to some allergens associated with A. fumigatus than are healthy control horses.44 In addition, bronchoalveolar lavage fluid concentrations of IgE and IgG directed against antigens of A. fumigatus were greater in RAO-affected horses than in control horses,45 and extracts or whole preparations of A. fumigatus induced greater in vitro histamine release from degranulating mast cells of RAO-affected horses than from healthy horses.46 RAO has been extensively reviewed elsewhere.47,48
Keratomycosis
Aspergillus spp. are destructive pathogens in the eye. The propensity for horses to develop mycotic keratitis may be related to the fact that Aspergillus spp. and other fungi are usually among the commensal conjunctival flora, and horses are constantly exposed to fungi in fodder, bedding, and stable dust. A warm, humid environment (e.g., heated barn, ambient conditions) may predispose horses to fungal keratitis. Frequent treatment of bacterial keratitis with topical antibiotics may reduce the numbers of nonpathogenic bacteria while favoring multiplication of pathogenic bacteria and fungi.15,49 The use of ophthalmic antibiotics is associated with a shift in the population of ocular flora from predominantly gram positive to gram negative.50 Commensal flora of healthy eyes may produce antimicrobial substances that limit the population of pathogenic organisms.14,15,49 For example, Streptomyces natalensis, a conjunctival commensal organism in healthy horses, produces the antifungal natamycin.14,51 In one study, affected eyes of 32 of 39 horses with fungal keratitis (82%) were treated intensively with topical ophthalmic antibiotics before entry into the study, suggesting that this treatment may have been a risk factor for developing keratomycosis.26
Topical corticosteroids may enhance fungal replication and reduce corneal tissue resistance to fungal invasion, resulting in enhanced penetration of the cornea.25,52 However, Andrew et al.26 documented prior treatment with topical corticosteroids in only 6 of 39 horses with keratomycosis, whereas 5 of 23 affected horses had prior treatment with topical corticosteroids in another study.53 These findings collectively suggest that, although treatment with corticosteroids increases the risk of keratomycosis, other factors are likely important in the pathogenesis.
The equine eye is prominent, and the cornea has a large surface area, putting this structure at risk for trauma. Any epithelial defect can provide entry for fungi into the deeper layers of the cornea, or penetrating trauma may seed deeper layers of the cornea with fungi. Fungal replication and dead fungal hyphae induce a severe polymorphonuclear neutrophil (PMN, leukocyte) inflammatory reaction. Local release of proteases from fungi, leukocytes, and keratocytes destroys stroma.26,51,54 Once established in the corneal stroma, fungi tend to burrow toward Descemet’s membrane, where they are poorly responsive to medical treatment.51 Corneal avascularity and the absence of corneal lymphatics may slow the response to deep infection. Healing depends on neovascularization arising from the limbus. Stromal abscesses may result from penetrating corneal wounds that seed the deeper corneal tissues, although other factors may be important, particularly where clusters of cases are identified.51 A breach in Descemet’s membrane by a penetrating wound or fungal-induced inflammation may allow Aspergillus organisms access to the anterior chamber, resulting in endophthalmitis.
Deficiencies in the immunoprotective qualities of tear film and the cornea in some horses may predispose them to keratomycosis.28 Gliatoxin and other putative fungal virulence factors may inhibit corneal vascularization, reduce PMN cell infiltration, suppress cell-mediated phagocytosis, reduce cytolytic T-cell proliferation and activation, and decrease leukocytic antifungal proteinase activity.51 Aspergillus and Fusarium isolates from equine patients with keratomycosis inhibited angiogenesis in an in vitro model where fungi inhibited differentiation of capillary-like tubules from human umbilical vein endothelial cells.55 Corneal healing in horses depends on neovascularization by endothelial budding and subsequent corneal invasion of limbal vessels. Inhibition of this process could impair corneal healing.
Endometritis and Placentitis
Common factors that increase uterine exposure to the ubiquitous aspergilli include frequent uterine lavage (which may introduce fungal organisms), poor vaginal conformation, pneumonvagina, cervical adhesions, and urine pooling (see Chapter 8). Increased exposure to Aspergillus organisms increases the risk of infection.
Aflatoxicosis
Of the four types of aflatoxins, B1 is the most abundant.31 Aflatoxins are metabolized in the liver by mixed-function oxidases. One of the metabolites is a highly reactive oxide that binds with deoxyribonucleic acid (DNA), messenger ribonucleic acid (mRNA), and proteins, impairing protein synthesis and inducing cancer in some species.31 Impaired protein synthesis results in necrosis, particularly in the liver, and improper antibody formation. Acute intoxication with large doses of aflatoxin leads to liver damage and death. Chronic exposure to sublethal doses may be asymptomatic in horses or may induce immune suppression and ill thrift with poor feed conversion.56 Cumulative exposure increases the risk of liver and lung cancer in humans.56 Dietary deficiencies in protein, selenium, and vitamin E increase susceptibility to aflatoxicosis.31
CLINICAL FINDINGS
Mycotic Keratitis
Aspergillus spp. may colonize corneal erosions, producing lesions ranging from superficial abrasions with associated pain, miosis, blepharospasm, epiphora, and photophobia54,57,58 to severe interstitial keratitis of varying depths.57 These lesions usually develop rapidly, although indolent infections may occur.59 Focal or diffuse corneal opacity and edema may be present (Fig. 56-2). Ulcers may appear raised and are often characterized by roughened borders, with surrounding radiating lines of leukocyte infiltration.59 As fungi proliferate, dry, white to grayish, fluffy lesions may be observed, and the corneal surface may appear slightly green.59 As the fungi invade the corneal stroma, neovascularization, microabscesses, and stromal malacia may be observed. Secondary anterior uveitis with aqueous flare will ensue, and corneal rupture and endophthalmitis may be sequelae.59 Reliable signs that indicate healing of mycotic keratitis include clearing of the corneal edema, progressing from the periphery toward the lesion, in association with abundant, deep stromal vascularization that extends to the margin of the opacified stroma.24 As the stromal opacification decreases in size, it may become more dense because of fibrosis.
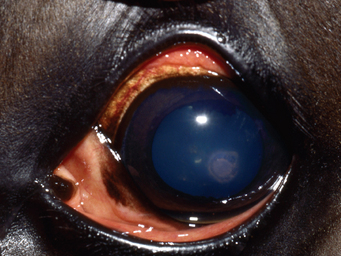
Fig. 56-2 Keratomycosis in a horse.
(Courtesy Dr. Anne Metzler, The Ohio State University College of Veterinary Medicine.)
Corneal stromal abscesses are characterized by a yellow-white opacity deep to intact (non–fluorescein-staining) corneal epithelium or a relatively small corneal defect51,58 (Fig. 56-3). Satellite lesions are often observed, and associated corneal edema may be prominent.
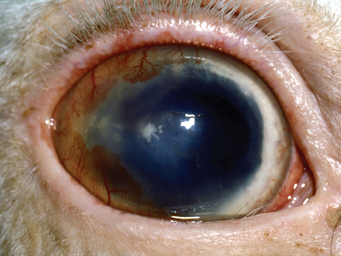
Fig. 56-3 Mycotic stromal abscess in a horse.
(Courtesy Dr. Anne Metzler, The Ohio State University College of Veterinary Medicine.)
Mycotic keratitis should be suspected in corneal lesions that respond poorly or worsen during antibiotic therapy and in those that show improvement followed by deterioration when treated with topical corticosteroids.60 Differential diagnoses include bacterial keratitis (especially caused by Pseudomonas spp. and β-hemolytic streptococci), equine recurrent uveitis, bacterial stromal abscesses, viral keratitis, corneal dystrophies or degeneration, and indolent ulcers.59
Pulmonary Aspergillosis
In the early stages of infection, clinical signs of respiratory disease may be absent or mild.40 Advanced or extensive pulmonary disease may be associated with nasal discharge, nasal plaques, abnormal breath sounds (crackles, wheezes, pleural friction rubs), tachypnea, dyspnea, and pleural hemorrhage.34,40 A history of vague respiratory signs that respond poorly to antibiotic therapy should suggest the possibility of fungal infection. In one retrospective study, 4 of 29 horses with pulmonary aspergillosis had a history of intermittent treatment with moderate doses of corticosteroids, and 23 of 29 (79%) had been previously treated with antibiotics.34
Nasal or Sinus Aspergillosis
Mycotic plaques caused by Aspergillus spp. may occasionally occur on the mucosa of the nasal passages in the absence of Aspergillus infection elsewhere, or these plaques may be present in horses with concurrent pulmonary aspergillosis. Three horses with unilateral aspergillosis of the middle meatus had unilateral, foul-smelling nasal discharge and intermittent epistaxis, and two of three had submandibular lymphadenopathy.61 Affected horses may have multiple mycotic plaques, and nasal discharge may be continuous or intermittent. In a retrospective and prospective study of 277 cases of sinonasal disease, 13 horses had intranasal mycotic lesions, mycotic sinusitis, or mycotic lesions at the sinonasal ostium.62 Most of these lesions were caused by Aspergillus fumigatus. Lesions of the nostrils were often ulcerative granulomas.63 An ulcerated mycetoma at the commissure of the upper lip of one horse was caused by infection with Aspergillus versicolor that extended through to the buccal mucosa.5
Central Nervous System Aspergillosis
Aspergillus niger caused necrotizing vasculitis with cerebral infarction in an 18-year-old Morgan mare with a 10-day history of diarrhea.4 The mare was depressed, incoordinated, and dysphagic and died after 4 days of treatment with fluids and trimethoprim-sulfamethoxazole and pyrimethamine.
Endometritis and Placentitis
Mares with fungal endometritis typically have a white to gray vaginal discharge. The uterus contains variable amounts of fluid that may induce distention and flaccidity.23,64 Aspergillus has also been reported to cause severe focal placentitis, resulting in abortion.65
Aflatoxicosis
Ingestion of aflatoxin (500-1000 parts per billion [ppb]) by mature horses decreased feed intake, with resultant weight loss and induced liver damage.7 In other horses, ingestion of feed containing 900 ppb or more than 6500 ppb of aflatoxin caused death, with brain, liver, kidney, and heart damage in affected horses.7 Aflatoxins, fed at doses greater than 2 mg/kg, were uniformly fatal in weanling ponies.66 Aflatoxin B1 was found at a concentration of 114 μg/kg in corn fed to horses that died (three) or became ill.67 Chronically affected horses show a spectrum of clinical signs, including weight loss, behavioral changes (somnolence, yawning, aggression, head pressing, circling, blindness) associated with liver impairment, or death.7
DIAGNOSIS
The diagnosis of aspergillosis must be based on identification of the organism in tissue, such as the cornea. The organism grows well on most commercially available fungal culture media.2 Isolation of fungi from the conjunctiva, proximal airway, or other tissues that could collect environmental contaminants is not sufficient to establish a definitive diagnosis. Culture as well as cytologic evaluation of infected tissue or exudates should be attempted. Specimens should be obtained from several areas of the lesion, including deeper tissues, to maximize the possibility of finding fungal organisms if they are present.
Serology
Attempts to diagnose invasive aspergillosis based on serology have been unrewarding. Anti-Aspergillus antibodies can be detected in healthy as well as diseased horses, likely because of constant environmental exposure to these fungi.34,42 Counter immunoelectrophoresis and enzyme-linked immunosorbent assay (ELISA) using complex antigenic mixtures did not discriminate between healthy and diseased horses.68 However, immunoblotting analysis based on reactivity to low-molecular-mass antigens, was positive in diseased horses but not in healthy horses of the same study. This assay is not commercially available.
Real-Time Quantitative Polymerase Chain Reaction
A recently developed, real-time (RT) quantitative polymerase chain reaction (PCR) test evaluates the number of copies of DNA from fungi in corneal tissues of horses with mycotic keratitis.69 Horses with confirmed fungal disease had a significantly greater number of copies of fungal DNA than horses with healthy eyes. Four of five horses with fungal keratitis had more than 1000 copies/25 ng of DNA, whereas healthy horses had 5 to 321 copies/25ng of DNA in their corneal tissue.69 This assay is commercially available at the author’s institution. This approach to diagnosis holds promise for quantifying fungal load and may prove useful to monitor response to therapy.
Culture
Confirmation of identification of the genus and species of the fungus requires culture of tissue or fluid samples. Aspergillus spp. will grow readily on most fungal media.2 Although cultures may be positive in as little as 3 days, an average of 25 days was required to identify the isolates in one study.70 It is not practical to wait for culture results before beginning treatment.
Susceptibility Testing
Antifungal susceptibility testing (measuring the inhibitory activity of the tested antimicrobial agent) and correlations between in vitro susceptibility and clinical outcome of invasive fungal diseases in human patients have been the subject of intensive research.70 Many factors other than susceptibility of the etiologic agent to the chosen drug affect clinical outcome, including host immune status, location of the infection, duration of the infection, drug pharmacokinetics, and patient compliance.71 Standardized methods of assessment of in vitro susceptibility of yeasts and filamentous fungi to some common antifungal drugs have been developed.72 Tentative “breakpoints” for fluconazole, itraconazole, and 5-fluorocytosine against Candida spp. have been established, and breakpoint values for fluconazole and flucytosine are useful in predicting clinical outcome.72 Meaningful correlations between in vitro susceptibility test results and clinical outcome for most filamentous fungi and yeasts have not been established.71–74
Current recommendations to physicians managing patients with fungal diseases are to (1) identify a fungal isolate at the genus and species level if possible; (2) perform in vitro susceptibility testing (using approved methods) only for fluconazole and flucytosine susceptibility of Candida isolates from sterile sites; (3) attempt susceptibility testing for Candida spp. and amphotericin B; Cryptococcus neoformans and fluconazole, flucytosine, or amphotericin B; and Histoplasma capsulatum and fluconazole for patients in whom initial antifungal therapy has failed; and (4) select therapy for all other fungal isolates based on guidelines or survey data.72,75
Antifungal sensitivity testing is difficult to obtain in the veterinary clinical setting,20 and results of in vitro susceptibility testing often do not correlate well with clinical response to treatment.28 In particular, fluconazole may demonstrate low activity with in vitro test systems, but high activity in vivo, possibly due in part to the drug’s excellent tissue solubility.76 Troke et al.77 demonstrated that fluconazole was 15-fold more potent than ketoconazole in a model of vaginal candidiasis in mice, despite being 80-fold less active in vitro. The value of in vitro antifungal susceptibility testing in veterinary medicine is unproven.
Currently, determination of fungicidal activities of antimicrobial agents against yeasts and molds holds promise for the development of clinically relevant correlates of in vitro susceptibility. In vitro studies employing minimum fungicidal concentration (best assessed in animal models) or “time-kill” methods (ability of antimicrobial agent to kill fungal isolate over time) have predicted in vivo response.78 To date, these methods have not yet been standardized, and their use in the veterinary setting has not been explored.
Biopsy
Aspergillus organism are readily identifiable in infected tissues. Typical hyphae in tissue are 2-5 μm in diameter and branch dichotomously at 45-degree angles (Fig. 56-4). Hematoxylin and eosin (H&E) stain will identify the hyphae, although periodic acid–Schiff (PAS) and silver stains, such as Gomori’s methenamine silver (GMS), are reported to be particularly useful in demonstrating the organisms.23,40
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


