CHAPTER 86 Artificial Insemination and Embryo Transfer in Sheep
Artificial insemination and embryo transfer in sheep offer many advantages for genetic improvement. They are not new techniques, but several advances in technology have made them somewhat easier for cost-effective implementation. At this time, many of the drugs that are routinely used for synchronization and superovulation are not available to U.S. veterinarians, yet they are commonly used in most foreign countries.
ARTIFICIAL INSEMINATION
Benefits
Selection and Management of the Ram
Two weeks before semen collection, rams should be individually housed to help prevent injury, avoid homosexual behavior (especially in ram lambs), and help control any special dietary needs of individual rams. This may also help more submissive rams increase their libido. All contact with ewes should stop at this time, but it is important to maintain ewes within sight, smell, and sound of the rams.
RAM TRAINING FOR SEMEN COLLECTION WITH THE ARTIFICIAL VAGINA
Electroejaculation for Semen Collection
Most common equipment includes either a Bailey or a Ruakura ram probe, although other probes by other manufacturers will also work. These types of probes have a simple on/off switch that delivers stimulation of 10 to 15 volts of 30 to 50 sine or square waves. Operators should thoroughly familiarize themselves with whatever equipment they are using and use it appropriately for the situation.
HANDLING, EXAMINATION, ASSESSMENT, AND EVALUATION OF SEMEN
Volume will range from 0.5 to 1.5 ml and may vary based on frequency and type of collection. Repeated collections over several days will decrease volume. Motility will normally be wave-like on gross examination under low power (10–50×) on a prewarmed slide without a coverslip. The motion is scored as in Table 86-1 and any sample scoring 2 or below is discarded.
Table 86-1 Scoring System for Wave Motion Under Low-Power Microscopy, 10× to 50×
| Score | Class | Description |
|---|---|---|
| 5 | Very good | Dense, very rapidly moving waves; individual sperm cannot be observed; >90% of the sperm are active |
| 4 | Good | Vigorous movement, however waves and eddies are not as rapid as those in score 5; 70 to 90% of the sperm are active |
| 3 | Fair | Only small, slowly moving waves; individual sperm may be observed; 40 to 65% of sperm are active |
| 2 | Poor | No wave motion forming, but some movement of sperm visible; 20 to 40% of sperm are active |
| 1 | Very poor | Less than 10% of sperm active; possible to observe slight “flickering” of sperm with poor motility |
| 0 | Dead | No movement apparent |
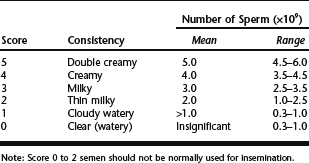
Concentration should be calculated accurately because a reliable number of sperm per insemination is critical to pregnancy rates. An average ejaculate will contain 3.5 to 6.0 billion sperm per milliliter. A hemocytometer, spectrophotometer, computer-assisted analysis, or other accurate method of counting sperm is best, but under some field conditions, concentration of the sperm may be estimated by consistency of the sample using Table 86-2. Thick, creamy samples contain more sperm than those of a more dilute, watery consistency.
Other tests that may be useful include a morphologic and acrosome integrity test using eosin-nigrosin stain or hypo-osmotic swelling tests. Individual sperm motility and progressive forward movement by dilution of the sample with extender, sodium citrate, or phosphate buffered saline. Longevity of sperm using various extenders and examination at set intervals (usually 1–2 hours) over time may be used to evaluate both the sperm and which extender gives greatest sperm life. Temperature and holding conditions must be carefully monitored to be sure that the sperm is not affected by outside factors and the evaluation is valid.
DILUTION AND INSEMINATION DOSE USING FRESH SEMEN
Freezing Semen
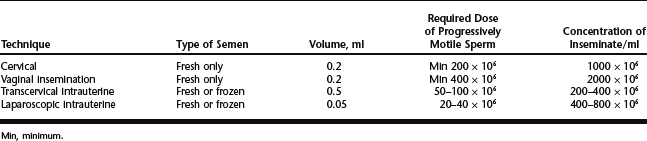
Freezing semen in straws is similar to freezing bull semen, but the extender, dilution rates, cooling rates, and other factors will vary from laboratory to laboratory. Ram semen is more difficult to freeze than bull semen and some rams (5–10%) will not freeze successfully with current techniques and extenders that are available. Normally ram semen is diluted to at least 1:8 with the chosen extender with final dilution depending on the type of insemination (laparoscopic AI versus cervical AI versus transcervical AI). Doses of semen for the various techniques can be found in Table 86-3.
Semen can be loaded into straws or cooled before loading into straws, then cooled slowly to 4° to 5° C over a period of 1.5 to 2 hours. Depending on the laboratory or extender, semen is then either frozen over nitrogen vapor (4 cm for 0.25-ml straws or 6 cm for 0.5-ml straws) on a chilled (5° C) rack or held for several more hours to allow equilibration and then frozen as described previously. After 8 to 10 minutes in the vapor, the semen is then plunged into the liquid nitrogen and from there loaded into canes and transferred to a tank for storage using standard techniques. When using a programmable freezer, chilled filled straws are loaded into the freezer and cooled at the following rate: 4° C per minute until semen reaches −12° C, then 40° C per minute from −12° to −40° C, then finally 50° C per minute from −40° to −140° C. After this point is reached, straws are loaded into canes and stored as described previously.