CHAPTER 17 Antioxidants and Horse Health
During the last two decades, the physiologic and pathophysiologic roles of oxidants and antioxidants have been intensively investigated in humans, laboratory rodents, and horses. Oxidants, which were initially considered potentially harmful metabolic byproducts, are now recognized as important cellular mediators and signaling molecules. Nevertheless, if the pro-oxidant burden overwhelms the antioxidant defense systems, oxidant-antioxidant disequilibrium or oxidative stress can develop. Biomarkers can be detected in body fluids, enabling assessment of oxidative stress in various physiologic and pathologic conditions. Antioxidants are recommended for maintaining optimal oxidant-antioxidant equilibrium as a means to prevent or treat oxidative stress-associated diseases, but the paucity of systematic research in this field limits the recommendations for therapeutic antioxidant use in equine medicine.
ROLE OF OXIDANTS AND ANTIOXIDANTS FOR HEALTH
Role of Oxidants
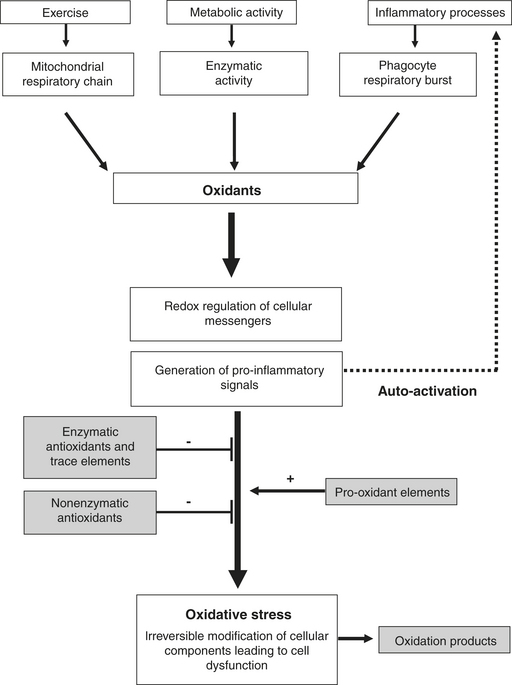
Initially oxidants were considered to be potentially harmful byproducts of cellular metabolism and part of the immune system. However, during the last two decades, oxidants have been identified as important messengers in numerous intracellular pathways. The implication of so-called redox signaling in activation of transcription factors and in mitotic and apoptotic processes indicates that oxidants are indispensable signaling molecules. The expression of inflammatory genes is also dependent on oxidation-reduction reactions, which makes oxidants potential pro-inflammatory stimuli (Figure 17-1).
Antioxidant Defense Systems
Exposure to oxidants from various sources has led organisms to develop a series of defense mechanisms, including preventative mechanisms, antioxidant defenses, and repair mechanisms. Prevention of oxidant generation occurs mainly within the mitochondrial respiratory chain, where enzymatic complexes strongly limit electron leakage. Furthermore, proteins that bind free iron and copper (which promote oxidative processes), such as transferrin, ferritin, ceruloplasmin, and albumin, further decrease the cellular capacity for oxidant generation.
Antioxidants are implicated in the inactivation or transformation of oxidants, which can either be transformed by antioxidant enzymes into less reactive forms or react with antioxidant molecules that are chemically stable. The most important antioxidant enzymes are superoxide dismutase (SOD), catalase (CAT), and glutathione-peroxidase (GPx). The catalytic activity of these enzymes allows transformation of superoxide anion into hydrogen peroxide (H2O2) and water, thereby inactivating important amounts of oxidants. Trace elements, such as selenium (Se), zinc (Zn), copper (Cu), and manganese (Mn), play an important catalytic role in the enzymatic activity of GPx (Se) and SOD (Zn, Mn, Cu). Antioxidant molecules of reduced molecular weight are the most numerous endogenous antioxidants. They can be divided into hydrophobic and hydrophilic antioxidants.
Oxidative Stress: A Consequence of Oxidant-Antioxidant Disequilibrium
Molecules that have undergone oxidation can be detected and are used as oxidation markers. Their presence can disturb cell function, especially with oxidative modifications to DNA, and can lead to mutations. Oxidation of proteins contributes to enzyme dysfunction and cell membrane lipid peroxidation, initiating chain reactions that can compromise cell integrity. Such cellular perturbations appear to be of particular importance in inflammatory conditions in which increased oxidant production can further enhance the inflammatory process through a positive feedback mechanism (see Figure 17-1).