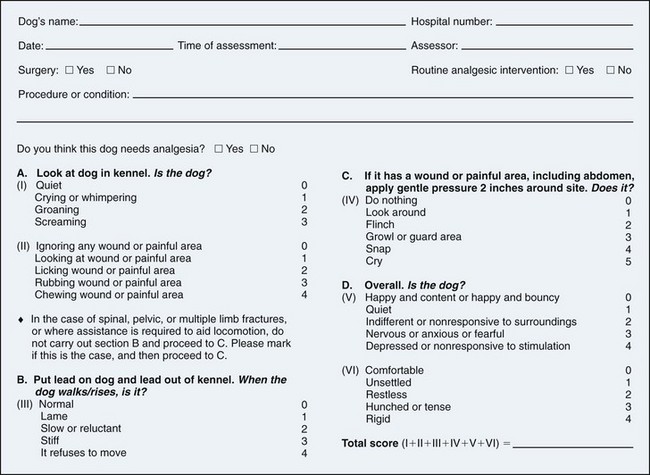
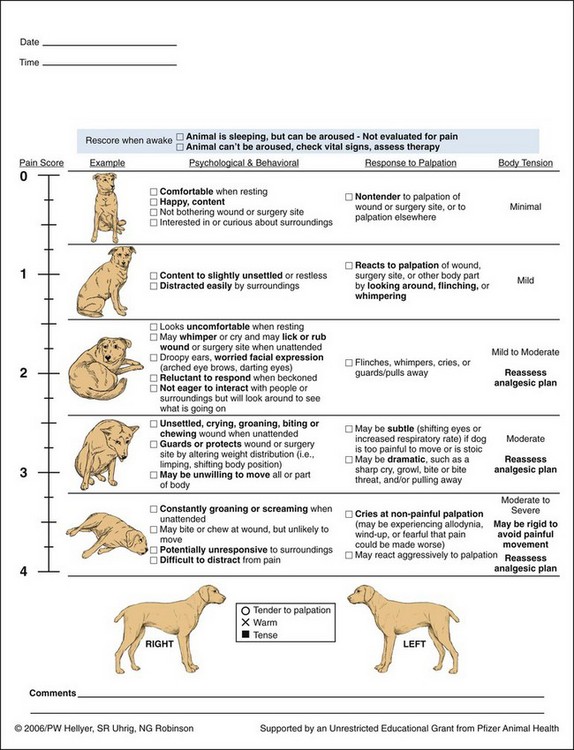
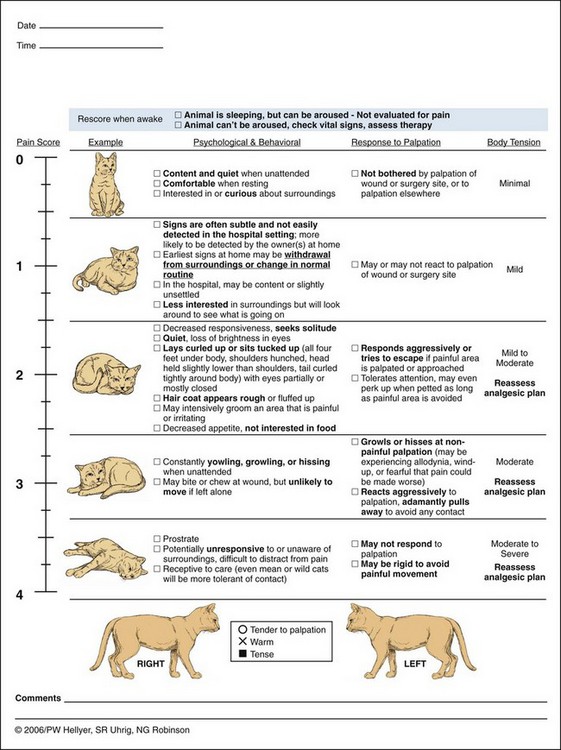
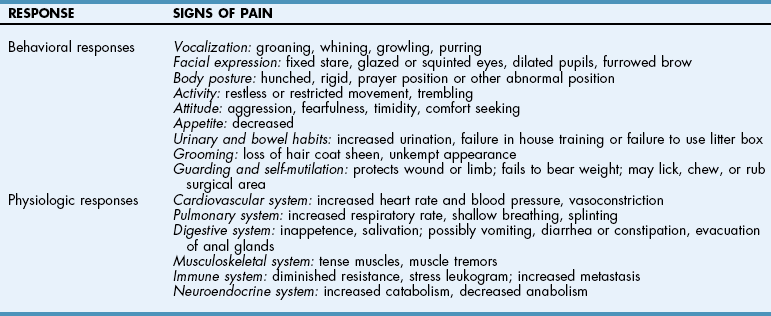
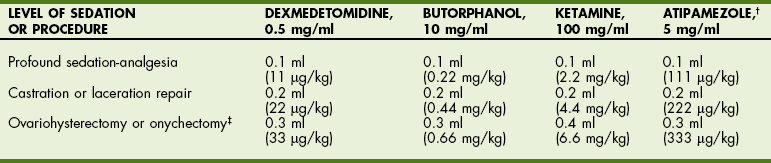
Chapter 12 Although preemptive analgesia has received mixed reviews in the human medical arena, ample evidence suggests that if it is used adequately, some benefit is derived. Several key techniques make preemptive analgesia more successful. First, the entire surgical field should be covered. Second, onset of action of medications must be taken into account and preemptive medications must be given far enough in advance of the surgical stimulus. Next, the analgesia must be adequate enough and deep enough to block transmission of pain pathways. And last, the analgesia must last long enough into the postoperative period to give adequate pain control (Macres et al, 2009). The efficacy of preemptive analgesia depends on its ability to blunt the “windup” of pain (the volley of afferent impulses in the spinal cord that result from stimulation of a nociceptor). A single preemptive intervention may adequately control initial operative pain responses. However, unless this is followed by adequate and occasionally prolonged postoperative pain control, central sensitization to pain can still occur. This underscores the necessity of adequate follow-up of pain control therapy. It is important to realize that one regimen will not work for all situations or all patients. A customized, multimodal approach is one of the best techniques for providing optimal analgesia for surgical patients in the operating room and during the postoperative period. Before any discussion of different modalities of pain control is presented, how to assess the level of pain in our animal patients should be discussed (Table 12-1). Numerous types of assessment techniques are available, and none is perfect. In the human patient, the visual analog scale is one of the most common pain scales currently in use because it allows the patient to assess his or her own pain and assign a number to that pain. However, this can be problematic even with patients who speak the same language as the caregiver. Patients have different tolerances and expectations of the surgical experience, and a 5 out of 10 pain level often has different implications for different people. These same assessment problems are magnified 10-fold with any attempt to assess pain in our veterinary patients. Therefore an understanding of how animals exhibit pain is very helpful in knowing when and how to treat the surgical patient. Behavioral and Physiologic Responses to Pain Modified from Carroll GL: Small animal pain management, Lakewood, Colo., 1998, American Animal Hospital Association Press. First and foremost, it is important for the veterinarian to find a pain scale that the staff may be able to utilize quickly, easily, and consistently. The role of a pain scale is to guide analgesic therapy, not to deny it (Mich and Hellyer, 2009). It can be helpful in providing timely and adequate pain management and in guiding the tapering of analgesic therapy. When choosing a pain scale guide, one needs to recognize each scale’s strengths and limitations. Two behavior-based pain scales stand out in being easy to use and having less bias. The Glasgow Composite Measure Pain Score (GLCMPS)–Short Form was developed for use in dogs in acute pain (Reid et al, 2005). Numbers from 0 to 4 or 5 are assigned to differing behaviors and are tabulated to create a score on which analgesic therapy can be based (Fig. 12-1). The two parts consist of initial observation followed by a leash walk and a hands-on portion. Potential advantages that make it more accurate than many scales include limited bias from the observer, ease of use, and specific behaviors identified as being present or absent. One significant disadvantage is that it was developed for dogs, but not for cats. Additionally, it has limited value in the immediate postoperative period because postanesthetic sedation is not part of the evaluation (Mich and Hellyer, 2009). A modified form of the Glasgow Pain Scale has been studied and was found to be effective in assessing acute pain in dogs (Murrell et al, 2008). In this study, sedation was incorporated into the evaluation. The second behavior-based pain scale that deserves mention is the Colorado State University Veterinary Medical Center Acute Pain Scale (Fig. 12-2). It was designed to be a user-friendly scale with verbal and visual descriptions. Similar to the GLCMPS–Short Form, it assigns numbers from 0 to 4 and has both observational and hands-on sections. A section for evaluating body tension is also included. This is the first scale designed to address the feline population (Fig. 12-3). Numerous choices are available for premedication of the veterinary patient. Before deciding which premedication to use, one first must decide what it is that needs to be accomplished with the premedication. Is it restraint of the aggressive animal? Is it part of the pain protocol in painful surgeries? Or is it necessary for calming an anxious patient? Once the goal of the premedication has been determined, then choosing one or more appropriate drugs is the next task. Fortunately, there are many premedications from which to choose, each with desirable and undesirable attributes (Box 12-1). Combining diazepam with opioids as a premedication attenuates the potential for excitability and produces more reliable sedation in many patients, especially dogs (see Box 12-1). Diazepam is often given at induction with ketamine, barbiturates, propofol, etomidate, or opioids. Its use in this combination takes advantage of the sedative and relaxation properties and allows reduced dosing of the other induction medications. As with other benzodiazepines, diazepam has minimal effect on the cardiovascular system. Additionally, very little respiratory depression occurs at lower doses. Tidal volumes will decrease with small increases in partial pressure of arterial carbon dioxide (PaCO2). These changes are more pronounced when other respiratory depressants are given with diazepam. Midazolam is a water-soluble benzodiazepine available for IM or IV injection; it does not cause pain with either route. Additionally, it has a rapid onset of action and rapid metabolism. These qualities make it a very good choice, especially when used as a premedication with an opioid in very young, elderly, or sick dogs and cats. As with other benzodiazepines, midazolam is metabolized by the liver and excreted by the kidneys. Smaller doses may be warranted in older or debilitated animals or in those with liver or kidney disease. In humans, midazolam is the most widely used premedication, given alone or with fentanyl. In veterinary medicine, it is useful especially in small mammals such as ferrets and rabbits and in some birds (Lemke, 2007). It is well tolerated in many dogs, especially when combined with other sedatives (see Box 12-1). Its short duration of action frequently makes it a better choice for induction with propofol, etomidate, ketamine, or barbiturates than diazepam in both cats and dogs. Acepromazine has been the most commonly used tranquilizer in veterinary medicine for many years. It can provide profound sedation at small doses, but side effects limit its usefulness in elderly, sick, or trauma patients. The widespread practice of administering acepromazine as a premedication and then maintaining anesthesia with an inhalant anesthetic (e.g., isoflurane, sevoflurane) with inadequate monitoring contributes to the high incidence of hypotension in small animal surgical patients (Lemke, 2007). Acepromazine has profound cardiovascular effects. It causes pronounced peripheral vasodilation while at the same time decreasing stroke volume and cardiac output. This combination can dangerously drop blood pressures in both awake and anesthetized patients. Although this may be tolerated in young, healthy patients, it can be devastating in hypotensive, hypovolemic, or critically ill patients. Because acepromazine is metabolized in the liver and excreted by the kidneys, it should be used very cautiously in patients with hepatic or renal dysfunction. It also causes marked relaxation of the splenic smooth muscle, leading to pooling of red blood cells in the spleen. This sequestration of red blood cells causes a drop in hematocrit of 20% to 30%. Even with low doses of acepromazine, these physiologic responses of decreased cardiac output, vasodilation, and decreased oxygen-carrying capacity mean that multiple organ systems will have a decrease in oxygen delivery. In sick patients with increased metabolic requirements and oxygen needs, this can be a deadly combination. For these reasons, acepromazine generally is recommended for use only in young and healthy patients. In this patient population, it can provide profound sedation that may assist in quieting animals both preoperatively and postoperatively (see Box 12-1). In small animal veterinary medicine, the alpha-2 agonists most commonly used are medetomidine and dexmedetomidine. Historically, xylazine was used for many years, but it does not have selectivity for alpha-2 versus alpha-1 receptors, as do medetomidine and dexmedetomidine. Medetomidine is a racemic mixture, with dexmedetomidine being the active isomer. Therefore dexmedetomidine is twice as potent as medetomidine. Selective alpha-2 agonists may be used as premedication in healthy small animals for their sedative, analgesic, and muscle relaxant properties (Lemke and Creighton, 2010) (Table 12-2; see also Box 12-1). Even in low doses, they can augment the analgesic and anesthetic effects of other drugs. Both medetomidine and dexmedetomidine dosages are calculated using body surface area. This helps to reduce variations in sedative response from one type of body conformation to another. Premedication with 125 µg/m2 or 0.5-1 µg/kg has been shown to reduce the quantity of induction drugs required, as well as the inhalant agent needed for maintenance of anesthesia. Constant rate infusion (CRI) and microdoses of medetomidine and dexmedetomidine have been used with success as part of balanced anesthetic protocols to reduce isoflurane requirements (see Box 12-2). A comparison of 1, 2, and 3 µg/kg/hr determined that a CRI of 1 µg/kg/hr, after a 2.5-5-mg/kg loading dose, had the most stable hemodynamics (Uilenreef et al, 2008). Dexmedetomidine-Butorphanol-Ketamine Combination in Healthy Cats* *Based on a 4.5 kg (10 lb) cat. All drugs (except atipamezole) can be mixed in one syringe and administered as a single IM injection. If given IV, the drug doses in this combination should be halved; however, if a deeper plane of anesthesia is desired, the full IM dose can be given IV. ‡More invasive procedures should have a stronger opioid chosen for postoperative analgesia. From Ko JC, Knesl O, Weil AB, et al: FAQs: analgesia, sedation, and anesthesia: making the switch from medetomidine to dexmedetomidine, Compend Contin Educ Vet 31(Suppl 1A):1, 2009. The cardiovascular effects of dexmedetomidine and medetomidine are dose dependent and biphasic, with the initial phase of hypertension and reflex bradycardia lasting approximately 15 to 20 minutes after administration. This is followed by a decrease in sympathetic tone, resulting in vasodilation, hypotension, and bradycardia. Monitoring of blood pressure and heart rate is recommended. Before bradycardia is treated, blood pressure should be taken. If necessary, an anticholinergic (e.g., atropine, glycopyrrolate) may be used to treat the second phase of bradycardia when hypotension is also present. It has been shown that a marked hypertensive response occurs when atropine is given before dexmedetomidine (Alvaides et al, 2008). Because of this marked hypertension, anticholinergics are not recommended before alpha-2 agonists are administered or during the hypertensive phase (Ko et al, 2009). Because of blood pressure changes associated with these drugs, they are best used in healthy dogs and cats. Alpha-2 agonists are best avoided in hypotensive, hypertensive, hypovolemic, elderly, or critically ill patients. Respiratory function is maintained, but urine output and blood glucose are increased with both medetomidine and dexmedetomidine. The alpha-2 agonists are reversible with yohimbine and atipamezole. Both reverse the sedative and analgesic effects. Atipamezole as an IM injection has been used to treat the bradycardia associated with alpha-2 agonists and probably is the drug of choice in hypertension-induced bradycardia. Once given, the sedative and analgesic effects are gone as well, which may interrupt or complicate the procedure. With life-threatening bradycardia and hypertension, atipamezole can be given as a single slow IV bolus (off-label) at a rate of 5 to 20 µg/kg IV over several minutes (Ko et al, 2009). This should be done only in an emergent situation. Atropine and glycopyrrolate are anticholinergic medications often added to the premedication arsenal. With an increase in monitoring abilities, the routine addition of this class of drug to the premedication regimen is often unnecessary. Both atropine and glycopyrrolate act at parasympathetic cholinergic sites to increase heart rate and decrease salivation. Atropine has a greater effect on heart rate as compared with glycopyrrolate. However, glycopyrrolate has a slightly greater effect on decreasing oral secretions compared with atropine. Atropine and/or glycopyrrolate should be easily available for intravenous treatment of bradycardia in the anesthetized patient. At very low doses, a paradoxical decrease in heart rate can occur after administration. This occurs because of inhibition of presynaptic cholinergic receptors that are part of the sympathetic pathways (Stoelting and Hillier, 2006). This is more likely to occur with atropine than with glycopyrrolate because of the greater anticholinergic effects of atropine on the heart and because of its rapid onset of action. Usually this paradoxical bradycardia quickly resolves, but if necessary the dose can be repeated to cover more cholinergic receptors and obtain the desired cardiac effect. Glycopyrrolate and atropine are used with the anticholinesterases in reversing muscle paralysis from nondepolarizing muscle relaxants. The anticholinesterases can cause severe bradycardia that can lead to sinus arrest. Therefore an anticholinergic is given intravenously right before the anticholesterase or is mixed in the same syringe to be given together. Glycopyrrolate has a similar onset of action as neostigmine, whereas atropine has a similar onset of action as edrophonium. Propofol is an insoluble drug that requires a lipid emulsification. Soybean oil is used for the oil phase, and egg lecithin as the emulsifying agent (Stoelting and Hillier, 2006). Because this solution can support bacterial growth, it is recommended to discard unused portions after 6 hours. Propofol quickly crosses the blood-brain barrier and exerts its action centrally on GABA receptors. Propofol causes rapid hypnosis, but no analgesia, when given as an intravenous bolus. It is metabolized in part by the liver and is excreted in the urine. However, propofol clearance exceeds hepatic blood flow, emphasizing redistribution of propofol into other tissues, including the lungs. Neither liver nor kidney disease appears to affect the clearance rate of propofol. The use of ketamine, not as an induction agent but as part of a multimodal approach, has increased in both veterinary and human medicine. It reversibly binds to NMDA receptors in the dorsal horn as an antagonist. Low doses of ketamine and CRI take advantage of this NMDA receptor binding. Ketamine helps with somatic more than visceral pain, but in a multimodal protocol with infusions that begin before surgery and last into the postoperative period, ketamine provides additional visceral analgesia over opioids alone (Himmelseher and Durieux, 2005). In veterinary patients, CRI has been shown to reduce isoflurane requirements while increasing heart rate and blood pressure under general anesthesia (Pascoe et al, 2007). Patients undergoing major procedures with significant surgical trauma or those patients with preexisting central sensitization may benefit from perioperative opioid-ketamine infusions (Lemke and Creighton, 2010). Ketamine produces cardiovascular effects that resemble sympathetic nervous system stimulation with increased heart rate and blood pressure, cardiac output, and cardiac oxygen demand. Ketamine should be avoided in tachycardic, hypertensive, subaortic stenosis, hypertrophic cardiomyopathy, or sympathetically depleted patients. Because ketamine directly stimulates the sympathetic nervous system, chronically ill patients may experience a decrease in cardiac output and blood pressure, reflecting depleted catecholamine stores. Dramatic drops in heart rate and blood pressure can occur at induction, especially if higher doses of ketamine are used. As an example, this may occur with an animal that has been severely dyspneic for an extended period. The increased stress and work of breathing can deplete catecholamine stores. Therefore, ketamine may not be the best choice as an induction agent despite the fact that respiration is maintained. Thus, chronically ill patients may benefit more from low-dose or CRI ketamine rather than the higher dose required for induction (see Box 12-2).
Anesthesia and Perioperative Multimodal Therapy
Review of Pain Pathways
Pain Assessment
![]() TABLE 12-1
TABLE 12-1
Premedications
Benzodiazepines
Diazepam
Midazolam
Acepromazine
Alpha-2 Agonists
![]() TABLE 12-2
TABLE 12-2
Anticholinergics
Induction Medications
Ketamine
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Veterian Key
Fastest Veterinary Medicine Insight Engine