CHAPTER 7 Evaluating, Designing, and Accessing Herbal Medicine Research
An ongoing debate among herbalists and natural therapists involves what role, if any, science must play in the future of herbal medicine. Some feel that the traditional basis of herbal medicine provides a completely adequate therapy and that the scientific investigation of herbs or herbal therapy has little to offer. They caution that the wholesale incorporation of scientific methods into the practice of herbal medicine will result in adverse changes—changes that will make herbal medicine less than what it is today. They fear that herbal medicine will lose its traditional basis, its insight, and its soul. Perhaps it will become a sick hybrid that is neither scientifically sound nor valid as a therapy; possibly, herbal medicine will become totally reductionist, with herbs, similar to many modern drugs, used only for superficial symptom control. Among some herbalists, science is seen as a technique for information gathering that is inferior to the knowledge derived from insight, inspiration, and intuition.
Used properly and in context, good science has much to offer. But what is the proper context for herbal medicine? Phytotherapy has been defined as the positive incorporation of science and tradition. In particular, scientific investigation is useful for providing the solid, factual, background information that any therapist needs. For example, science can tell us that Ginkgo biloba is good for circulation, or that Hypericum perforatum is a valid treatment for depression. However, traditional considerations will often be more relevant in guiding the phytotherapist regarding when to apply this information in a clinical situation. In this context, science is just one tool to be used in the consulting room. During a consultation, a good practitioner will assess the patient, whether human or animal, as an individual, using insight, logic, and common sense and supported by the appropriate use of scientific information. The treatment of the patient as an individual can never be outweighed by results of double-blind clinical trials.
DESIGNING RELEVANT HERBAL MEDICINE RESEARCH
Phytopharmacologic Discovery
Why should phytochemicals have biological activity in humans? Baker suggests an evolutionary kinship (Baker, 1995). Enzymes in animals can share a common ancestry with enzymes or proteins in plants. Phytochemicals that are substrates of a plant enzyme may also be capable of being substrates of the corresponding human enzyme. In Baker’s examples, phytochemicals interact with enzymes that metabolize animal hormones, leading to hormone-like effects. One of the best examples of this is glycyrrhetinic acid from licorice, which exerts a potent mineralocorticoid effect without ever interacting with mineralocorticoid receptors.
Herbs are complex. It was difficult enough to understand exactly how a drug like aspirin, a single chemical in use for more than 100 years, worked in the human body. The scientist who did so shared the Nobel Prize. In the case of a chemically complex herbal extract, the task is that much more difficult, perhaps even impossible with today’s technology and today’s one-dimensional approach to researching pharmacology and therapeutics.
Research techniques in phytopharmacology
Limitations of the In Vitro Test in the Context of Phytopharmacology:
During an in vitro test, an herbal extract comes into direct contact with test cells, as shown in the diagram (Figure 7-1). Often, levels of exposure are unrealistic. But the reality, after an herb is orally administered, is very different, as is represented in the next diagram (Figure 7-2).
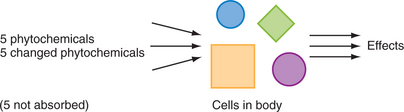
Figure 7-2 A diagrammatic representation of cellular exposure after oral ingestion of an herbal extract.
In other words, the cells of the body experience a modified version of the herbal extract and are often exposed to metabolites of the original compounds. Bowel flora play a special role here. In particular, some phytochemicals that are active in in vitro tests are not even absorbed. For this reason, extrapolation of in vitro research to whole body systems must be done with great caution. For more information on herbal pharmacokinetics and bowel flora metabolism of phytochemicals, the reader is referred elsewhere (Mills and Bone, 2000).
Examples of Phytopharmacologic Research on Humans and Domestic Animals:
Examples include the following:
Research Linking Quality and Efficacy
The issue of being able to relate the phytochemical content of an herb or herbal extract to its clinical efficacy is one of the potentially most fruitful areas of phytopharmacologic research but also one of the most complex, controversial, and difficult. Efficacy cannot occur without quality, but which phytochemicals in a plant define its quality from a therapeutic perspective?
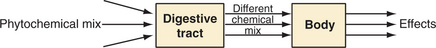
The patient is a black box when it comes to many herbs; we do not know what is happening in terms of relating the clinical outcomes to the phytochemical input (Figure 7-3). But as represented in Figure 7-4, we can break the “black box” up into two components, whereby the digestive tract acts as a filtering process and often an agent of change (as discussed previously). If a plant compound is not absorbed (or its metabolites are not absorbed), we can probably discount its relevance from the quality perspective.
Valerian—The hunt for the “active constituent”
The complex issue of relating the phytochemicals in a plant to its pharmacologic activity is well illustrated by research on valerian (Valeriana officinalis). Research is often too focused on hunting for the active constituent. The example of valerian shows that the research emphasis over the years has shifted from one phytochemical class to another (Schumacher, 2002). The question, still not conclusively answered in the case of valerian, is whether one class is more important than another (Mills and Bone, 2000) (Box 7-1).
BOX 7-1 Shifts in the Emphasis of Research
| Up to 1950s | Essential oil and alkaloids |
| 1960s | Valepotriates |
| 1980s | Valerenic acids and (maybe) valepotriate decomposition products |
| 2002 | Lignans (Schumacher, 2002) |
How to preserve or enhance quality in studies of therapeutic efficacy
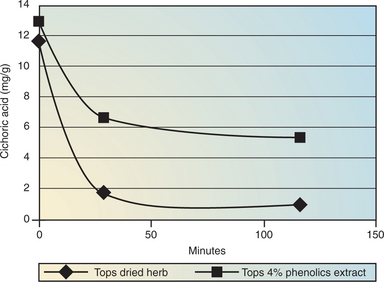
Referring to the first approach of preserving what is in the fresh plant as much as possible, does that mean there should be a preference for fresh plant tinctures? At the risk of being controversial, the postulated advantages of fresh plant tinctures are not supported by phytochemical fact. There is too much water in a fresh plant for the direct manufacture of a sufficiently concentrated preparation. However, there is another important concern. The high water content and the unquenched enzymatic activity mean that phytochemicals are being decomposed as the tincture is being made. Cichoric acid in Echinacea purpurea is now a well-known example of this (Bauer, 1989).
The experiment represented in Figure 7-5 was carried out in a simulated stomach and mimics what happens when equivalent quantities of Echinacea tops are ingested either as just the dried herb in a capsule or as an extract in a capsule (Lehmann, 2002). Cichoric acid levels degrade to almost zero for the dried herb, indicating that enzymatic activity is still present.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree