Conc. (μg/μl)a
Volume (μl)
Stereotaxic co-ordinates (mm)b
Strain
Sex
Reference
16
4
n/r
n/r
M
(31)
4
4
AP = +5 (to occipital structure), ML = −2.2, DV = −3.5
Swiss
M
(32)
4
4
AP = +0.5, ML = −2.4, DV = −3.1
C57Bl/6
M/F
(33)
3.32
4
AP = +4.8 (Lambda), ML = −1.7, DV = −3.0 and −2.7 (dura)
MNRI
M
(34)
4
4
AP = +3 (to occipital structure), ML = −2.2, DV = −3.5
Swiss
M
(35)
4
4
AP = +0.5, ML = −2.4, DV = −3.1
C57Bl/6
M
(36)
2
2
AP = +0.4, ML = −1.8, DV = −3.5
C57Bl/6
M
(37)
3
2 × 2
(1) AP = +1.0, ML = −2.1, DV = −2.9(2) AP = +0.3, ML = −2.3, DV = −2.9
C57Bl/6
M
(38)
3
2 × 2
(1) AP = +1.0, ML = −2.1, DV = −2.9(2) AP = +0.3, ML = −2.3, DV = −2.9
C57Bl/6
M
(38)
3
2 × 2
(1) AP = +1.0, ML = −2.1, DV = −2.9(2) AP = +0.3, ML = −2.3, DV = −2.9
C57Bl/6
M
(39)
6
n/r
AP = +0.8, ML = −1.0, DV = −2.5
A/J
M/F
(40)
3
3.9 μg or 5.4 μg
AP = −1.2, ML = ±1.1, DV = −5.0, with incisor bar set at ±0.0
CBA
F
(6)
2.5
2 × 2
AP = +0.5, ML = +2.4, DV = −4.0 and −3.0 (dura)
C57BL/6J/OlaHsd
M
(41)
3
2 × 2
(1) AP = +1.0, ML = − 2.1, DV = −3.2(2) AP = +0.3, ML = −2.3, DV = −3.2
C57Bl/7
M
(42)
2
2
AP = + 0.4, ML = −1.8, DV = −3.5
C57Bl/6
M
(43)
4
2
AP = + 0.8, ML = −1.9, DV = −2.6
C57Bl/6
M
(44)
2
1
AP = + 0.4, ML = − 1.8, DV = − 3.5
C57Bl/6/SWISS/sv129
M
(45)
As the focus of this chapter is on the unilateral mouse model and its deviation from the standard rat model, the readers is advised to refer to the instruction on rat surgery (discussed in Chap. 13) with respect to making up the toxin.
1.
The surgery suite needs to be equipped for mouse surgery, including anaesthesia, analgesia, heated recovery blanked/box, scalpel (size 10) and suture (thread 5.0), drill, micro-drive pump, syringe and lesion cannula.
2.
A Hamilton syringe is connected to the lesion cannula via polyethylene tubing. The Hamilton syringe is driven by a micro-drive pump.
3.
Make up 6-hydroxydopamine to the required concentration (calculate from free-base weight).
4.
Place mouse in induction chamber to induce anaesthesia (using 1–2% Isoflurane in a mixture of 2:1 oxygen to nitrous oxide as carrier gas (see Note 1)).
5.
Shave head and place animal into the stereotaxic apparatus.
6.
Clean the head and preferably swap with antiseptic (e.g. iodine).
7.
Make incision with scalpel along the midline of the animals’ skull.
8.
Using the scalpel, scrape away dura and connective tissue so that skull surface can be seen clearly.
9.
Locate bregma on the animals head and place the tip of the drill on bregma and adjust co-ordinates on the stereotaxic apparatus and gently burr hole.
10.
Load lesion cannula with 2–3 μl of fresh toxin and gently lower lesion cannula through the burr hole to the required depth and infuse the lesion site slowly with the toxin and leave needle in place to allow the toxin to diffuse for additional 3 min.
11.
Slowly remove lesion cannula to prevent drawback of the toxin, clean the animals’ head and carefully suture up to ensure swift recovery.
12.
Provide analgesia (Metacam s.c. injection or Paracetamol (1 mg/ml) added to the drinking water) and where necessary gather advice from local veterinary.
2.2 Post-Operative Care
Post-operative mortality is generally much higher in mice compared to rats receiving a similar lesion, as the result of aphagia and adipsia from both the decline in general health and spontaneous turning behaviour in the home cage; however, this can be overcome by intensive animal husbandry (see Note 1). It is essential to provide wet food/mash and injections of glucose-saline if required and monitor the animals’ weight on a daily basis for 14 days post-lesion surgery. Housing mice 2 per cage following surgery is also helpful to prevent bullying and facilitate feeding. Bullying in mice is common after surgery and if noticed mice should be separated permanently.
3 Assessment of the Lesion and Resulting Motor Impairments
3.1 Drug-Induced Rotation
As described in chapter by Dunnett and Torres, this volume animals that receive unilateral dopamine lesions to the nigro-striatal pathway will respond with a typical turning behaviour when challenged with various drugs that modulate the dopamine system (8–10). Dopamine losses of 90% or more in the rat relate to a peak rotation rate of 5/7 turns per minute following an amphetamine or apomorphine challenge (11–13), quantified with the use of automated rotometer bowls. In mice, a 31.5-fold change in directional bias is seen following an apomorphine challenge (1, 5). Generally, amphetamine- and apomorphine-induced rotations have been shown to correlate highly with the percent of TH positive cell loss from the SNpc and are useful in determining the severity of a SNpc 6-OHDA lesion in mice (7). Non-pharmacologically induced, spontaneous rotation in a novel environment in mice was first noted by observations in an open field environment (14), where dopamine released by mild anxiety causes turning toward the ipsilateral direction.
3.2 Recording Rotational Behaviour in Mice
1.
The video recorder is set up on a tripod facing downward above the laboratory floor.
2.
Each mouse is placed in standard laboratory beakers (H: 14 cm, D: 11.5 cm) and allowed to explore environment for 20 min. Mice are injected with 0.05 mg/kg apomorphine (s.c.) or 2.5 mg/kg methamphetamine (i.p.) and placed back into the beaker (see Fig. 1b). Drugs are dissolved into physiological saline and used immediately. Apomorphine is dissolved with the addition of 0.2% ascorbate and kept on ice in the dark before use.
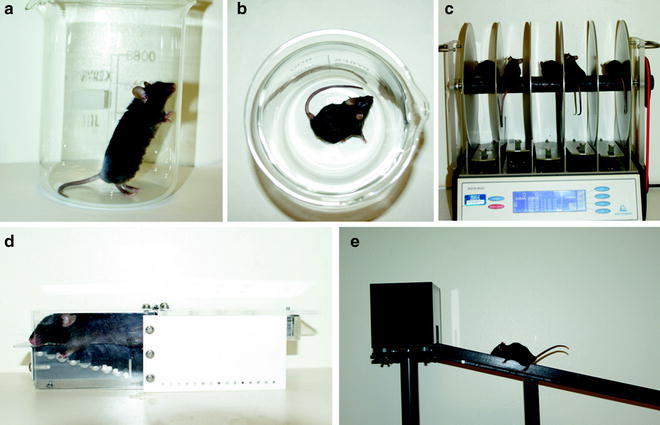

Fig. 1.
Behavioural tests to assess 6-OHDA lesion-induced deficit in mice. (a) Cylinder test. (b) Apomorphine-induced rotation. (c) Rotarod. (d) Staircase. (e) Elevated beam.
3.
The investigator will then record peak rotation over 20 min immediately following apomorphine injection and 20 min after methamphetamine injection (see Note 2 for variations in the dose and administration procedures that can be used for each drug).
4.
These recordings then can be analysed post hoc and full rotations can be counted using fast-forward video playback reporting net rotations over the period (No. of clockwise rotations – No. of anticlockwise rotations).
3.3 Assessment of Motor and Sensory-Motor Deficits
Unilateral dopamine loss in the 6-OHDA lesioned rat model produces a number of deficits (sensory, motor and cognitive) on the contralateral side including spontaneous circling toward the (ipsilateral) lesioned side, reduced use of the contralateral forelimb and a neglect of the contralateral side. For general behavioural considerations, see Note 3. The unilateral lesioned rat is simplistic, flexible and allows for unilateral, partial and full denervation of the nigro-striatal tract, enabling its suitability for a diverse range of motor, cognitive and therapeutic strategy driven tests (9, 15–18). We describe below a selected range of behavioural hand tests that assess these deficits in mice. There also remain a number of rat tests used for the assessment of unilateral lesion motor deficits that are not easily translated to the mouse (see Note 4).
3.3.1 Elevated Beam Test
The elevated beam test analyses the motor coordination and dexterity of right and left hindlimbs and forelimbs (see Fig. 1e). In beam-based studies, deficits have been reported in hemi-parkinsonian rats, mice transgenic for Huntington’s disease (19) and of late, unilateral lesioned mice (Heuer and Smith, unpublished observations). Here, foot slips are recorded on ipsilateral and contralateral sides on an inclined slope of decreasing width, until a designated point just before the animal reaches an enclosed platform at the top (for specific construction details, see Note 5). A foot slip is defined as paw misplacement where all digits slip off or entirely miss the ledge of the beam causing a slouch in the body posture of the mouse.
1.
The elevated beam is placed on a bench top such that the investigator can walk around the apparatus and see both sides.
2.
Training day 1: mice are placed pointing outward on the end of the elevated beam and have to balance and turn to face inward. Each mouse is required to do this three times.
3.
Training day 2: each mouse is positioned to balance on the inner ledge to traverse the beam to at least to the half way point three times. Mice are also placed in the enclosed space at the top of the beam and allowed to explore it for 3 min.
4.
Training day 3: mice must traverse the whole beam and reach the enclosed platform at the top twice.
5.
On one side of the beam, a video recorder is set up on a tripod to record foot slips on this side to be scored post hoc.
6.
Test day: mice are required to traverse the beam three times without turning in the opposite direction after the initial turn. The investigator must be on the opposite side of the elevated beam to the video recorder to count foot slips on that side, record the time to transverse the beam and note the direction of the initial turn.
7.
For analysis, the two quickest times over a designated 80 cm distance of beam and the corresponding foot-slip counts to these runs are averaged (foot-slip counts = No. of ipsilateral − No. of contralateral). Hindlimb and forelimb counts can be added together or used independently depending on the experimental question.
3.3.2 Skilled Reaching/Staircase Test
The staircase test, see Fig. 1d, has been developed for objective assessment of independent forelimb use in skilled reaching behaviour (20). The rat staircase apparatus has been scaled down from the rat version and validated for use in the mice (21). The testing apparatus consists of a home box that is connected to a narrow corridor, just wide enough for the animal to crawl into, but not wide enough for the animal to turn around. In the corridor, a double staircase with eight steps per side is separated by a central platform. The animal can climb on the platform and reach down to the steps which are baited using 20 mg precision sucrose pellets. With the exception of the first two steps, pellets can only be retrieved via grasping by the respective arm. Outcome parameters that are assessed during a 15 min session are: total number of pellets eaten; total number of pellets displaced and side bias in the amount of pellets retrieved (for general considerations regarding the use of staircase test, see Note 6).
1.
Food restrict animals to 85–90% of their free-feeding weight at least 1 week prior to starting behavioural testing maintaining them at this weight for the duration of testing (see Note 3).
2.
On the first day of training only, bait both staircases with as many pellets as possible whilst on all other testing days the steps are baited with two 20 mg precision sucrose pellets per step.
3.
Leave the animals for 15 min before putting them back into their home cages.
4.
Count and record the number of pellets remaining per step and side.
5.
Train the animals on a regular basis until the number of pellets eaten reaches asymptote.
6.
Clean apparatus before commencing testing other animals in the same apparatus.
7.
Present data as number of pellets eaten (total and side bias); number of pellets replaced (replace but not eaten); maximum distance reached (last step with two or more pellets). Side bias can be expressed as (Pellets retrieved left – Pellets retrieved right)/(Pellets retrieved left + Pellets retrieved right).
3.3.3 Cylinder Test in Mice
Mice can also be tested for paw preference in a novel cylindrical environment (6, 7). A beaker (H: 14 cm, D: 11.5 cm) is placed in front of two mirrors at 90° so that a 360° view can be seen by the observer so that the left/right forelimb bias is recorded (see Fig. 1a). As with the rat protocol (see Fleming and Schallert, this volume) the session is videotaped and scored post hoc by detailed freeze frame analysis. Simultaneous ipsilateral and contralateral forelimb touches were excluded and mice failing to reach 20 touches were removed from the cylinder after 10 min. Mouse models with lesions to the MFB, SNpc and Striatum typically express a 70% usage of the ipsilateral limb correlating to greater than 85% dopamine depletion (6); comparable to levels recorded in the rat.
3.3.4 Corridor
The corridor test is used to assess deficits in sensory-motor function and proprioception. The mouse is placed in one of the two corridors, with sugar pellet baited pots placed at intervals along the length of the passageway. The apparatus is ergonomically modified for the mouse 2× (60 × 4 cm) from rat-based models (15). An ipsilateral bias of 75% is seen in the mouse when using the corridor correlating with near complete neuronal degeneration of the Striatum and SNpc (7) comparable with deficit levels recorded in the rat (15, 22). As with the rat test (see Fleming and Schallert, this volume), the forelimb bias is noted for the first 20 retrievals from sugar pellets placed in wells along the side of the corridor in either parallel pairs or staggered.
1.




Prior to testing, mice are maintained at 85–90% of their free-feeding body weight (problems occurring with the food restriction of mice are discussed in Note 3).
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


