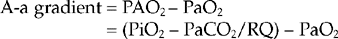6 Manifestations of Septicemia
Case 6-1 Foal with Septic Pneumonia
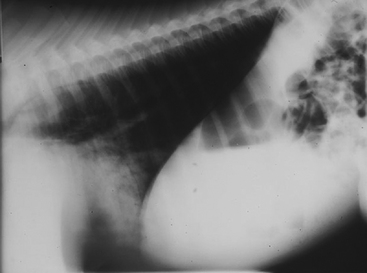
On presentation, ’03 Hufflepuff was markedly depressed (Figure 6-1). The foal had a poor suckle reflex and bright pink mucous membranes with a capillary refill time of two seconds. His temperature was 100.3°F, heart rate was 112 beats per minute, and respiratory rate was 48 breaths per minute with an increased respiratory effort (excessive rib excursion). Auscultation revealed harsh broncho-vesicular lung sounds, without other abnormalities. The remainder of the physical examination was considered normal.
HISTORY AND PHYSICAL EXAMINATION
The respiratory system of the neonatal foal is particularly vulnerable to injury. In one retrospective study, 50% of all foals presented to a referral hospital had some form of pulmonary involvement.1 Foals with respiratory dysfunction also showed the highest mortality rate. This was reflected in a recent postmortem study of equine neonates, where the respiratory system was the most common system affected.2
Pneumonia as a clinical manifestation of sepsis shares similar historical findings as a foal with sepsis, as described in Chapter 5. Colostrum leakages prior to parturition or colostrum deprivation (poor colostrum quality or quantity or delayed intake) are risk factors of bacterial pulmonary disease in neonatal foals, as they are in sepsis.3 In the current case, a delayed and inadequate colostrum intake was probably the result of partial rejection of the foal by an inexperienced mare. Infectious neonatal respiratory diseases are usually part of complex multiorgan systemic infections (sepsis), commonly precipitated by failure of passive transfer (FPT).4 Administration of 1 liter of intravenous plasma at 18 hours of age resulted in adequate immunoglobulin levels upon admission of the foal, but may have only been partially protective due to the foal’s exposure to facultative pathogens prior to effective immune protection.
Early in life, localizing clinical signs of respiratory disease may be absent even in the presence of extensive disease. Dyspnea may be seen in affected foals and manifest as an increase in respiratory rate and effort. After the first deep breaths taken at birth, foals should breathe with a subtle chest excursion. As respiratory disease progresses, one might notice signs of increased breathing effort by an excessive movement of the intercostal muscles and an increased abdominal component to respiration. Finally, thoraco-abdominal asynchrony (paradoxical breathing) may ensue in foals in respiratory distress, fatigue, or failure. However, the initial signs of respiratory distress and hypoxemia are frequently vague. Even some severely hypoxemic foals may only show restlessness, and considerable resistance and struggling when being handled or restrained. Abnormal respiratory sounds (crackles or wheezes) may be heard on auscultation. However, even normal foals may show crackles on the down lung after having remained in lateral recumbency for a prolonged period of time. Furthermore, foals with no auscultable abnormalities may still have extensive pulmonary disease. Cough and nasal discharge are usually absent in the early stages of neonatal pulmonary disorders.5
Cyanosis is a sign of severe hypoxemia (Figure 6-2). The arterial PaO2 (partial pressure of oxygen), however, must reach very low levels (35 to 45 mm Hg) before clinical cyanosis is observed. Approximately 5 g/dl of unoxygenated hemoglobin in the capillaries generates the dark-blue color that is appreciated as cyanosis in humans.6 Therefore, severely anemic foals may never appear cyanotic even in the face of profound hypoxemia. Weakness, depression, anorexia, weak or absent suckle reflex, dehydration, and fever may also be noted in foals with respiratory disease. In this case, evidence of depression, weakness, and injected mucous membranes should alert the clinician of possible underlying sepsis.
PATHOGENESIS
Respiratory disease and dysfunction in neonatal foals may present as a primary condition or may occur secondary to other disease processes.7 Equine neonatal pneumonia is generally defined as inflammation of the pulmonary parenchyma in foals less than four weeks of age. The etiologies of pneumonia in the newborn foal include systemic inflammatory response syndrome (SIRS), hematogenous spread of infection, aspiration of foreign material, and inhalation of airborne pathogens.5 Hematogenous (ascending) infections occur in association with bacteremia or sepsis, which may be acquired in utero from a placental infection or perinatally through environmental contamination (e.g. omphalitis, omphalophlebitis).
Perinatal pulmonic infections are more common in foals that are immunocompromised due to failure of absorption of maternal antibodies (FPT). An immature ciliary apparatus and the presence of fewer alveolar macrophages in neonates in comparison to adult horses lead to decreased bacterial clearance from the lungs.8 Reduced complement values in neonates may further contribute to decreased humoral defense against invading bacterial infections. If colostrum intake is insufficient and immunoglobulin G levels remain low, the foal is not only deprived of specific antibody protection, but neutrophil function is also seriously impaired.9
E. coli, Klebsiella spp, Actinobacillus equuli, Salmonella spp, and Streptococcus spp are some of the more common bacteria involved in neonatal foal pneumonia.5,10 Equine viral arteritis (EVA) and herpes viral infections (EHV 1 and 4) have been implicated in in utero viral infections.11 Although involvement of adenovirus in the pathogenesis of pneumonia has also been reported in a Thoroughbred foal, fatal adenoviral pneumonia is primarily associated with combined immunodeficiency (CID) in Arabian foals.12,13 Additionally, a recent report documents the isolation of influenza A virus from a seven-day-old foal with bronchointerstitial pneumonia.14 Rare opportunistic fungal pathogens may include Pneumocystis carinii (renamed Pneumocystis jiroveci in humans), Aspergillus, Candida, and Mucor spp in immune-deficient foals.
Descending respiratory infections may be related to inhalation pneumonia (transmission of viral, bacterial, or fungal airborne pathogens), aspiration of infected amniotic fluid due to placental infection, aspiration of gastric reflux, iatrogenic aspiration (oil, medication, oral supplements), and aspiration of milk and meconium (see Case 8-3). Aspiration of meconium occurs in utero in foals that experience fetal distress. Though the meconium is commonly sterile at this time, it creates mechanical airway obstruction, surfactant inactivation, and pulmonary inflammation (caused by vasoactive mediators, chemotactic and inflammatory cytokines, including tumor necrosis factor α, interleukin 1β, and interleukin 8).15 The resulting edema and vasoconstriction may lead to hypoperfusion of the pulmonary parenchyma, with damage to type II pneumocytes and decreased production of surfactant.16 This type of “secondary surfactant deficiency” may also be induced by other forms of generalized pulmonary inflammation, including diffuse bacterial pneumonia.
Neurologic dysfunction, severe inflammation, or structural abnormalities of the upper airways predispose to aspiration pneumonia postpartum (see Case 8-3). Descending infection secondary to bacterial colonization of the mucosal surfaces of the oropharynx and nasopharynx rarely occurs, except when other predisposing factors are present.
DIAGNOSTIC TESTS
Bloodwork
As seen in the previous chapter, the effect of sepsis on the circulating neutrophil count is variable. Acute neutropenia, with or without a left shift, is commonly related to bacterial sepsis or endotoxemia when there is overzealous production of cytokines. With the appropriate stimulus, neutrophilia with or without a left shift may be present. Profound lymphopenia may be found in acute viral diseases as well as sepsis and endotoxemia.17 Lymphocyte counts are generally higher than neutrophil counts in the fetus and may reflect the degree of immaturity in premature foals. Physiologically, neutrophil counts in neonatal foals increase to mean values of approximately 8 × 103 neutrophils/μl during the first 24 hours postparturition, due to endogenous glucocorticoid steroid release. Lymphocyte counts may physiologically decrease to mean values of 1.4 × 103 lymphocytes/μl a few hours after birth, and then gradually increase to 5 × 103 lymphocytes/μl at three months of age.18 Neonatal respiratory disease is not consistently related to specific abnormalities in the serum biochemistry analysis, but may depend upon underlying conditions such as sepsis, SIRS, or perinatal asphyxia.
Blood Gas Analysis
Arterial blood gas analysis is preferentially used to determine oxygenation and ventilation in neonates. Although pulse oximetry provides a continuous measure of arterial oxygen saturation, this technique has not been rigorously tested in the foal, and accurate readings are often difficult to obtain in the awake foal. Technically, an arterial blood sample can be obtained from any artery where a pulse can be felt. The more common sampling sites include the dorsal metatarsal artery located between the third metatarsal (cannon) bone and the cranial ridge of the forth metatarsal (lateral splint) bone of the hind limb; the branchial artery on the medial aspect of the elbow; the femoral artery at the external inguinal ring; the carotid artery, which runs medial and deep to the jugular vein; and the transverse facial artery, which is located caudal to the lateral cantus of the eye (ventral to the zygomatic arch). Successful arterial sampling is somewhat dependent upon the cardiovascular status of the foal. It is difficult to feel a pulse at the more distal sites in foals that are hypotensive or hypothermic. Additionally, peripheral oxygenation may not be equivalent to partial oxygen pressures obtained from central arteries.
Clipping and applying alcohol to the site of arterial puncture may help to visualize the artery. It is best to use a 22- to 25-gauge needle and a special blood gas syringe that contains an anticoagulant (e.g. sodium heparin) to obtain your sample. These syringes are usually self-filling. Once the sample has been drawn, all air bubbles should be evacuated from the syringe and the syringe should be capped to prevent exposure to room air. Room air will falsely increase the true PaO2 and decrease the PaCO2. Samples should be analyzed as soon as possible. If there is a delay (>10 minutes), then the sample can be refrigerated for two hours without significant problems. New handheld blood gas analyzers make this a practical test even in farm settings. Table 6-1 and Figure 6-3 are guidelines for the interpretation of arterial blood gas results in equine neonates.
Table 6-1 Arterial Blood Gas Interpretation
| Parameter | Rounded Mean Normal Values (mm Hg) | Age of Foal Postpartum* |
|---|---|---|
| PaO2 | 55–60 | 30 minutes (lateral recumbency) |
| 70–80 | 6 hours (lateral) | |
| 70–80 | 12 hours (lateral) | |
| 70 (lateral) to 90 (standing) | 24 hours | |
| PaCO2 | 50–55 | 30 minutes (lateral recumbency) |
| 43–47 | 6 hours (lateral) | |
| 43–47 | 12 hours (lateral) | |
| 42 (standing) to 50 (lateral) | 24 hours | |
| Caution | ||
• Mild hypoxemia may be observed in foals after prolonged recumbency (especially if peripheral samples are taken). • Changes in body temperature result in large changes in PaO2, PaCO2, and pH without affecting HCO3. A low body temperature decreases PaO2 and PaCO2 while raising pH. • Air contamination in the ABG sample will falsely increase measured PaO2 and decrease PaCO2 levels. | ||
* Summarized from Harvey JW: Normal hematologic values, In Koterba AM, Drummond WH, Kosch PG, eds: Equine clinical neonatology, Philadelphia, 1990, Lea & Febiger.
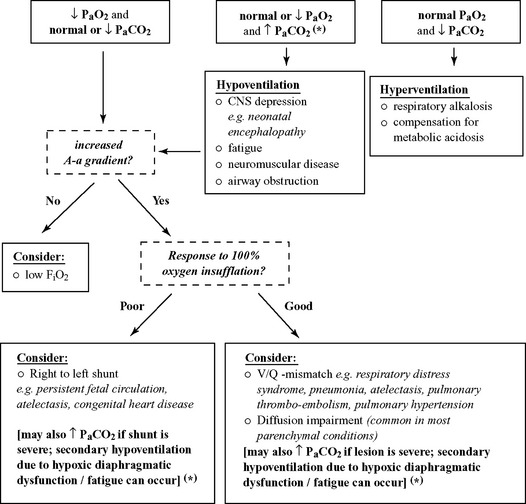
In the absence of alterations in the inspired oxygen fraction (FiO2), arterial hypoxemia may result from four basic processes, including hypoventilation, diffusion impairment, ventilation-perfusion inequality (VQ mismatch), and shunt (intrapulmonary and extrapulmonary shunt).19
Alveolar hypoventilation is defined as a reduced volume of inspired air reaching the alveoli per unit time, and is always related to an increase in PaCO2 (hypercarbia). Conditions associated with alveolar hypoventilation include drug-induced respiratory depression (morphine, barbiturates), brain stem disease, abnormal respiratory muscle function (botulism, diaphragmatic hernia, dysfunction, or fatigue), thoracic cage abnormalities (rib fractures), increased airway resistance (upper and lower airway obstruction) and pleural space disease.20 Although the mainstay of hypercarbia is hypoventilation, hypercapnic respiratory failure may also be multifactorial and can be exacerbated by severe VQ mismatch, shunt, or diffusion impairments. However, hypercarbia in foals with respiratory failure due to consolidating pneumonia, atelectasis, ARDS, etc., is more likely related to hypoxic diaphragmatic failure (secondary hypoventilation) than VQ mismatch, shunt, or diffusion impairment.
Impaired diffusion results from an increase in the blood-gas barrier. Since carbon dioxide diffuses more readily than oxygen, the PaCO2 is usually not increased in conditions that only cause impaired diffusion, until the lesion is severe. Diffusion impairments may be seen in conditions such as pulmonary edema, pneumonia, atelectasis, or pulmonary contusion.20
The adequacy of pulmonary gas exchange is also determined by the balance between pulmonary ventilation and capillary blood flow, which is commonly expressed as the ventilation-perfusion (VQ) ratio. A VQ ratio above 1 describes conditions in which ventilation is excessive relative to capillary blood flow (dead space ventilation). In normal human subjects, dead space ventilation accounts for 20% to 30% of the total ventilation. Dead space ventilation increases when the alveolar-capillary interface is destroyed (e.g. emphysema), when blood flow is reduced (e.g. low cardiac output, pulmonary thrombo-embolism, persistent pulmonary hypertension), or when the alveoli are overdistended by positive pressure ventilation. A VQ ratio below 1 describes the condition in which capillary blood flow is excessive relative to ventilation (pulmonary edema, pneumonia, atelectasis, etc.).21
Shunting, on the other hand, is defined as any mechanism by which blood that has not passed through ventilated areas of the lung is added to arteries of the systemic circulation.20 In normal human subjects, intrapulmonary shunt flow (venous admixture) represents less than 10% of the total cardiac output.21 Rose and colleagues calculated the extent of physiologic shunt in neonatal foals and reported values of 16% to 18% in newborn term foals restrained in temporary lateral recumbency.22 In contrast, a 15% true shunt in adult humans is generally regarded as requiring oxygen therapy and 30% as life-threatening.23
Increased intrapulmonary anatomical shunting can result from pulmonary artery to venous fistulas or severe lung lobe consolidation. Cardiac shunting (right to left shunt) may be related to congenital heart disease (e.g. tetralogy of Fallot, patent ductus arteriosus) or persistent fetal circulation (PFC). It is important to note that hypoxemia that is unresponsive to oxygen therapy is suggestive of shunt.20
Alveolar to Arterial Oxygen Gradient
A simplified formula of the A-a gradient in animals’ breathing room air is
A-a gradient = 150 − (PaCO2/0.8) − PaO2
The normal A-a gradient is less than 15 mm Hg in healthy foals’ breathing room air, and increases with VQ mismatch, shunt, and severe diffusion impairments. The normal A-a gradient increases 5 to 7 mm Hg for every 10% increase in the inspired oxygen fraction in humans (FiO2 = 21% in room air). This effect is presumably caused by the loss of regional hypoxic vasoconstriction of the lungs after oxygen administration.21 Normal A-a gradients in foals receiving supplemental oxygen have not been established.
Radiographic Examination
Thoracic radiography is often helpful in establishing the presence of respiratory disease and in determining the type and extent of pulmonary involvement.7 In contrast to noninvasive and invasive pulmonary function testing (PFT, see below), thoracic radiography is used extensively in the clinical setting. Neonatal radiographic evaluation of the chest is facilitated by classifying the image appearance into radiographic patterns of pulmonary disease. Three major lung patterns (interstitial, alveolar, and bronchial patterns) are commonly used to define evidence of radiographic respiratory disease in neonatal foals. Although the vascular pattern may be helpful in assessing pulmonary perfusion, venous congestion, and cardiac anomalies, it plays a lesser role in the assessment of foal pneumonia.
Interstitial Lung Pattern
The pulmonary interstitium is composed of a connective tissue framework containing the pulmonary vasculature, lymphatics, nerves, and bronchi. Inflammatory infiltrate that affects these structures may produce radiographic changes that are referred to as the interstitial pattern. The hallmark of the interstitial pattern is an increase in background opacity, which results in the loss of visualization of the fine vascular structure of the normal, well-aerated lung.24
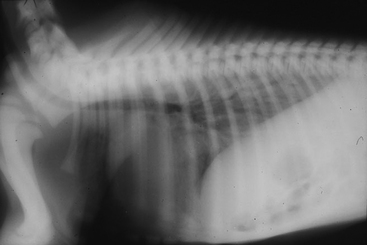
The interstitial pattern is the most common radiographic abnormality in foals with pulmonary disease. It may be associated with bacterial or viral pneumonia, pulmonary edema, meconium aspiration, lung immaturity, acute lung injury, and prolonged recumbency due to dependent atelectasis.25 Compression of the lung by distended abdominal viscera may also result in an apparent increase in the interstitial pattern due to decreased lung inflation (Figure 6-4).
Bronchial Lung Pattern
Although the bronchi are a component of the interstitial complex, diseases of this system are often considered separately. Radiographic signs of bronchial disease include increased thickness and density of the bronchial walls, or changes in bronchial lumen diameter (e.g. bronchiectasis). A bronchial lung pattern is more suggestive of chronic disease. However, a peribronchial pattern indicates infiltrates surrounding the bronchi and may be seen in acute and chronic inflammatory conditions.24
Alveolar Lung Pattern
The alveolar or air-space pattern is characterized by displacement of air from the alveoli by transudate, exudates, or blood, leading to a relatively homogenous increase in soft-tissue opacity. Air bronchograms may appear as lucent branching structures within an opaque lung field. The finding of air bronchograms is an indication of nearly complete alveolar filling.24 Edema, aspiration, infection, hemorrhage, and pulmonary consolidation commonly produce an alveolar pattern in neonatal foals (Figure 6-5).26 Additionally, a diffuse air-space pattern can result from respiratory distress syndrome (RDS) in the neonate, acute lung injury (ALI), and acute respiratory distress syndrome (ARDS) in the equine neonate.27
A recent study has documented that radiographic abnormalities involving the caudodorsal lung region were most common in foals admitted to a referral center.28 Bedenice and colleagues showed that the presence of dyspnea, tachypnea, a fibrinogen concentration >400 mg/dl, SIRS, hypoxemia, or FPT in neonatal foals may be predictors of underlying respiratory disease and should prompt an early radiographic evaluation of the thorax, even in the absence of other localizing clinical signs.3,28
’03 Hufflepuff was tachypneic upon presentation and showed diffuse radiographic infiltrates with a predominance of caudodorsal distribution. This is consistent with previous findings that tachypnea most reliably related to diffuse (caudodorsal, caudoventral, and cranioventral) pulmonary changes.28 The presence of underlying sepsis or SIRS in this foal may further explain the predominant caudodorsal pulmonary infiltrates. Pulmonary ventilation and perfusion is closely matched in horses, and the percent regional blood flow is increased in the caudal lung lobes.29 Additionally, Amis and coworkers documented that ventilation and perfusion follow a vertical gradient and increase in the dorsal lung regions of horses.30 SIRS may initiate severe vascular leakage and thus contribute directly to the pathogenesis of lung injury in regions of increased relative blood flow.28
Computed Tomography
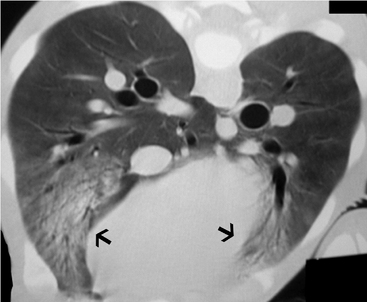
Additionally, computed tomography (CT) may serve as an important and sensitive means of thoracic imaging in anesthetized patients. Computed tomography has played an important role in improving our knowledge of the pathophysiology, morphology, and progression of pulmonary dysfunction in humans.31 It is not only a research tool, but an invaluable technique that allows for the quantitative and qualitative characterization of structural lung abnormalities, assessment of lung structure–pulmonary function relationships, the effect of ventilation strategies (e.g., PEEP), and therapeutic maneuvers (e.g., patient position) on lung structure and the progression of pulmonary disease (Figure 6-6). However, the use of CT in equine neonatal pulmonary disease is currently restricted to referral centers.
Diagnostic Sampling
Diagnostic sampling of the respiratory tract is less commonly performed in neonatal foals compared to adult horses. Transtracheal aspirates (TTA), broncho-alveolar lavage (BAL), protected catheter brush (PCB), or protected aspiration catheter sampling (PAC) is useful in stabilized patients to direct antimicrobial therapy and evaluate therapeutic efficacy. Generally, the clinician will depend upon the blood culture results to direct antibiotic therapy in the critically ill foal with respiratory compromise. Although rare in foals, thoracocentesis may also be of diagnostic and therapeutic importance in cases of pleuropneumonia and chylothorax. Lung biopsies are rarely performed and are limited to patients with chronic disease or suspected fungal pneumonia.
Pathophysiology
The term lung injury is generally used to describe the pulmonary response to a wide range of injuries occurring either directly to the lung or as a consequence of injury or inflammation at other sites in the body.32 Lung injury is a common sequela to sepsis, aspiration, or trauma and may be clinically difficult to differentiate from primary pneumonia, ARDS or RDS in premature foals (see Case 8-1).
The pathophysiology of ALI is driven by an aggressive inflammatory reaction. In the exudative or acute phase, injury to the alveolar epithelial barrier leads to an increase in permeability to protein and extravasation of solutes and water. Pathologically, diffuse alveolar damage with neutrophils, macrophages, erythrocytes, protein-rich edema fluid in the alveolar spaces, hyaline membranes, capillary injury, and disruption of the alveolar epithelium may be evident.33
ALI is diagnosed in humans if acute respiratory symptoms, a PaO2/FiO2 ratio <300 mm Hg, and noncardiogenic diffuse radiographic infiltrates occur following direct lung injury (e.g., aspiration, lung contusion, diffuse pulmonary infection) or indirect lung injury (e.g., sepsis, severe nonthoracic trauma, hypertransfusion). ARDS represents the more severe form of lung injury and is associated with a PaO2/FiO2 ratio <200 mm Hg in humans.34 Mortality rates from ARDS in humans have ranged from 40% to 60% in the past with the majority of deaths being related to sepsis or multiorgan dysfunction rather than primary respiratory causes.33 More recent studies indicate a reduced mortality of 36% and 34%.35