5 Septicemia
Case 5-1 Septic Foal
Bugs Bunny, an 18-year-old Quarter Horse mare, had an uneventful fourth pregnancy, with the exception that she developed edema in the udder and the caudal dependent abdomen in the two weeks prior to parturition. All of her previous pregnancies were uncomplicated with parturition occurring between 345 and 350 days gestation. Her previous foals did well. Bugs Bunny foaled on Bermuda grass pasture in central Georgia in a gentle rain at 1 am on March 13, 2002, at 360 days gestation. The mare was fitted with a Foal Alert™ monitor (Foal Alert, Atlanta, GA) and thus foaling was attended by the owners. Thirty minutes after presentation of both forelegs and the head, foaling had not progressed. The owners assisted delivery of the foal by traction on the forelimbs. The foal was able to attain sternal recumbency in several minutes and had a suckle reflex (Figure 5-1). The mare stood within 15 minutes of foaling. Bugs Bunny passed her placenta within one hour, and the owners reported that it appeared grossly normal and intact.
PATHOGENESIS OF INFECTION IN NEONATES
Infection, defined as the presence of microbes (bacteria, viruses, or fungi) inducing a host response,1 is often cited as the most common cause of illness and/or mortality in foals in the perinatal period. However, the actual incidence of perinatal infection in the general equine population is not well-known, relative to other noninfectious causes of morbidity or mortality. “Serious” infection in the first 90 days of life was reported in 37 of 132 foals (28%) on a Standardbred farm in New York over the 1986 foaling season.2 In contrast, in Stoneham’s survey of Thoroughbred stud farms during three breeding seasons (1988 to 1990) in the United Kingdom, only 11 of 406 foals (2.7%) were reported to have infection in the first four weeks of life.3
In terms of neonatal mortality, the role of infection is more obvious: septicemia was the reported cause of death in 26% of 334 foals less than 10 days of age born on a large farm in 1994 in Canada.4 In this review, sepsis was only marginally outnumbered by deaths caused from starvation or exposure (27%). In an extensive review of 3,514 pathology case reports in Kentucky from 1986 to 1991 on aborted fetuses, stillbirths, and foals that died less than 24 hours after birth, the most frequently cited cause of death was fetoplacental infection, which accounted for one third of the total cases.5 A large-scale study of 167 farms in Texas in 1991 involving 2,468 foals revealed that septicemia was responsible for 30% of the deaths in foals less than seven days of age.6 Geographical factors, management practices, and lack of detailed farm records likely account for the wide variation in the reported incidence of morbidity and/or mortality from perinatal infection in the general foal population.
In tertiary care centers, however, perinatal infection is consistently reported as one of the most common reasons for referral. At the University of Florida and the New Bolton Center at the University of Pennsylvania, blood is cultured from all foals that are admitted into their intensive care units. The reported incidence of positive blood cultures from these populations of foals are 26% and 28%, respectively.7,8 At the University of Georgia, from 1986 to 2000, a review of 507 records on all foals that were less than two weeks of age revealed that 250 foals were ultimately diagnosed with infection, based on a positive blood culture, positive sepsis score, or clinical or postmortem evidence of infection in three or more tissues.9 Considering that half of all admitted foals in the later study had evidence of significant infection, special attention needs to be focused on earlier recognition. Foal owners, care providers, and veterinarians should be familiar with historical events or prevailing conditions during the perinatal period that increase the likelihood of acquiring infection.
Considering the main ports of bacterial invasion, the skin, respiratory and intestinal mucosae, and umbilicus, the intestinal tract may actually serve as the most welcoming route of invasion. In the first hours of life, the cells that line the newborn foal’s small intestine are uniquely poised to engulf colostral immunoglobulins. However, it appears that this process is not discriminate to immunoglobulins, as other large (synthetic) molecular weight compounds can be equally absorbed by pinocytosis during this early postpartum period.10 Additionally, lack of tight junctions between intestinal cells during this period can freely allow large molecules to pass into the lymphatics.11,12 Although not directly proven, this period of time during which colostral absorption occurs could hypothetically allow bacteria to cross the intestinal barrier, into the interstitium, lymphatics, and bloodstream.11
In light of these potential exposures, it has been suggested that the risk of microbial invasion by the oral route can be reduced if mares foal on pasture or a well-kept, clean, and disinfected stall. The mare’s perineum, hindquarters, and udder should be washed and disinfected postpartum after passage of the placenta, and the foal should not be allowed to udder seek until this task has been accomplished.11 It has also been suggested to manually strip the mare’s colostrum, after disinfection of the udder, and to bottle or tube feed the foal prior to the foal contacting the mare. These practices have been shown in some situations to successfully prevent neonatal infection, even in foals with failure of passive transfer (FPT)11 or in foals whose dams were shedding Salmonella in their feces.13 The actual success of some of these recommendations is not entirely known. Although one large study involving 167 farms showed that the incidence of diarrhea was significantly lower in foals that were born on pasture compared to those born in stalls, the incidence of septicemia was the same despite foaling location.6
Avoidance of extensive wetness with disinfection of the umbilical cord is also recommended and is the premise for use of ethanol or 80% ethanol/0.5% chlorhexadine solution in human infants.14 In foals, chlorhexadine solution (0.5%) appears to be superior to 2% iodine or Betadine solutions.15 Harsh disinfection with tincture of iodine or Lugol’s solution can cause tissue necrosis, and neither of these should be used. Often cord disinfection is repeated several times in the first day or first few days postpartum, but attention should be focused on avoiding excessive desiccation or dampness of the umbilical cord.
THE INNATE IMMUNE SYSTEM
A key player in the ultimate battle between microbial colonization and establishment of infection is the innate immune system.16–18 Unlike adaptive immunity, which provides targeted defense against specific antigens that is magnified with each subsequent exposure, the innate immune system is comprised of components that provide unconditional defense, and therefore relies on immediate and less specific recognition of invading microbes. Neonatal foals are capable of initiating directed and specific responses against microbes, however it is the lag in the adaptive response that makes foals particularly susceptible to infection and more dependent on the innate immune system, compared to adult horses. A main feature of the innate immune system that enables immediate discrimination is pattern-recognition receptors (PRRs) that are capable of detecting a variety of microbial ligands, referred to as pathogen-associated molecular patterns (PAMPs).17 These ligands are evolutionally conserved molecules that are unique to microbes, are often shared by a broad range of organisms, and are usually essential for microbial survival or virulence. PRRs may be present on host cell membranes, in the circulation, or on mucosal surfaces, and they may be constitutively expressed and/or are released or secreted during the acute response to microbial invasion.
Examples of PAMPs include bacterial cell wall extracts, such as endotoxin, peptidoglycan and lipotechoic acid, and prokaryotic DNA. There is a complex and overlapping arsenal of PRRs, including the family of cationic antimicrobial peptides, such as defensins; collectins, such as mannose binding lectin; enzymes; integrins; and some of the acute-phase proteins. Ultimately, the interaction of a PRR with its PAMP can result in direct neutralization of the PAMP or microbe, or it may activate other components of the host immune system to deploy further defense mechanisms, initiate an inflammatory response, or commence tissue repair.16,17
A notorious PAMP in septicemia is endotoxin, with its respective mammalian host PRRs, lipopolysaccharide-binding protein (LBP), CD14, and Toll-like receptors (TLRs).19–21 Endotoxin is released from the outer cell membrane of Gram-negative bacteria during either rapid bacterial replication or upon bacteriolysis. It is comprised of three components, a long polysaccharide outer chain, a short polysaccharide core, and lipid A, which is the toxic moiety. Once in the blood, endotoxin’s amphipathic properties cause it to form aggregates that resemble micelles. LBP is a plasma constituent that serves as a PRR and efficiently extracts monomers of endotoxin from its circulating aggregrates and shuttles it to various locations.19 Through its interaction with lipid A, LBP effectively imprisons endotoxin, with its potential for toxicity determined by the complex’s final destination. LBP can rapidly deliver monomers of endotoxin to the surface of host inflammatory cells to evoke an inflammatory response, or it can be transferred to other neutralizing lipoproteins, such as high-density lipoprotein, for eventual removal from the blood. Thus LBP may serve to both enhance and hinder the biologic activities of endotoxin.
Once at the cell surface, endotoxin is transferred to cluster differentiation antigen 14 (CD14), a well-conserved PRR that is attached to the cell membrane by a glycosylphosphatidylinositol anchor.21 Mononuclear phagocytes (monocytes and macrophages) express abundant CD14, though other inflammatory cells also express minute amounts. CD14 is a 53 kDa glycoprotein that exists as both a cell membrane receptor (mCD14) and as a soluble form (sCD14) in the circulation, which is essentially identical to membrane-bound CD14 minus the glycosylphosphatidylinositol anchor. Similar to LBP’s roles, sCD14 is normally present in the circulation and has dual functions. It may bind and neutralize circulating endotoxin, thereby competing with membrane CD14. However, it may also enhance endotoxin’s toxic effects by transferring it to membrane CD14 or to cells that do not express membrane CD14. Another newly described source of CD14 is a smaller isoform found in extremely high concentrations in colostrum. This colostral CD14 is not present in the serum of foals prior to nursing, but it is readily detected after ingestion of colostrum.22 The exact role of colostral CD14 has not been fully elucidated, though if its roles are similar to sCD14, it may serve to either neutralize endotoxin or to alarm the host of bacterial presence.
Because CD14 does not structurally transverse the cell membrane, it must associate with a secondary protein, TLR, which contains a transmembrane portion that is capable of communication with the intracellular domain. CD14 binds to isolated monomers of endotoxin, intact bacteria, as well as several other PAMPS of Gram-positive bacteria. The name Toll-like receptor was adapted because of homology with a receptor found in Drosophila, called “Toll” that is responsible for dorsal-ventral polarity and innate immunity in fruit flies.20 Approximately a dozen TLRs have been identified, but TLR type 4 (TLR4) appears to be the isotype most important in the recognition of endotoxin, whereas TLR type 2 primarily confers recognition of Gram-positive bacterial components. Once the CD14-TLR4-endotoxin complex is complied at the cell surface, TLR4 requires a 160 amino acid helper molecule, MD2, to transmit a signal to the cytosol. Several intracellular signaling pathways have been reported to link a ligand-occupied PRR to cell activation, but the nuclear factor (NF) pathway is well characterized in its role for activating the promoter regions of inflammatory mediator genes.
Although endotoxin and activation of its PRRs explain how the innate immune system recognizes and responds to infection, it is only a one component of host defense. The innate immune system entwines several redundant levels of protection including the macroenvironment of physiologic barriers (skin and mucosa), the microscopic protection furnished by phagocytes (principally neutrophils, monocytes, and macrophages), and molecular defense (PRRs, immunoglobulins, complement, and acute phase proteins).17 The skin deters bacterial invasion by providing a physical barrier between the environment and deeper host tissue, in the form of host cells and normal flora. Other important components of resistance to colonization of the skin are an acidic pH, secretion of fatty acids, and the ability to “shed” cells.23
Like the skin, the mucosal surfaces of the gastrointestinal, respiratory, and genitourinary tracts provide a physical cell barrier that can desquamate, have normal flora, and abundant mucus that prevents colonization. The respiratory tract contains numerous “filters” that can trap bacteria that is inhaled, such as the turbinates, the long trachea, and ciliated respiratory tract lining cells. Mucosal epithelial cells in the gastrointestinal, respiratory, and genitourinary tracts secrete PRRs, such as defensins and collectins, which cause bacteriolysis and increase opsonization by phagocytes. Additional chemical defenses in the gastrointestinal tract include peroxidases, the acidic pH of the stomach, proteolytic enzymes, and lysozyme, all substances that can challenge bacterial cell wall integrity.23 Finally, physiologic activities such as sneezing, coughing, salivation, and peristalsis can also serve to remove potential pathogens.
Phagocytes, principally neutrophils, monocytes, and tissue-fixed macrophages, play a key role in innate immunity.17,23 In addition to engulfing and destroying bacteria via various oxidative, acidifying, and enzymatic mechanisms, neutrophils and monocytes secrete numerous substances, such as cytokines, growth factors, lactoferrin, and interferon, that can induce chemotaxis, enhance phagocytosis, cause bacteriolysis, or inhibit microbial replication.24 Although it has been shown that equine neutrophils are functional at birth, compared to neutrophils from adult horses, phagocytosis, oxidative burst activity, and killing are reduced in the first one to two weeks of life.25,26 These deficiencies are further exacerbated by FPT, as IgG and complement are needed for optimal phagocytosis of bacteria. Clearly these deficiencies in phagocytic activity in the newborn foal increase the susceptibility to microbial invasion in the perinatal period.
Lastly, the innate immune system is comprised of an extensive array of soluble molecules in the circulation, including PRRs, cytokines, chemokines, immunoglobulins, acute phase proteins, and other pro-inflammatory and anti-inflammatory mediators. Many of these molecular components are also present on cell surfaces, intracellularly, and on mucosal surfaces. Their overlapping presence in more than one tissue, the dynamic discovery of new molecules and rediscovery of new roles for previously identified molecules, confuses their classification. Although immunoglobulins are classically linked to adaptive immunity and anamnestic defense, they are a critical component for opsonization and the immediate defense against bacteria. The unique placental attachment in horses prevents placental transfer of immunoglobulins during gestation, thus foals are born agammaglobulinemic.10 Colostrum provides an immediate source of immunoglobulins, and failure of acquisition or absorption of colostral antibodies (FPT, see Chapter 3) in the first day of life has been well recognized as a major risk for infection.6,27–29 The fact that some foals with adequate serum IgG still acquire infection and that some foals with inadequate serum IgG do not acquire infection in the perinatal period2 supports the notion that the likelihood of infection is a combination of the risks of exposure and colonization, counterbalanced by the ability to defend from invasion. Regardless of inadequate serum IgG concentration, if exposure to microbes is minimized, then intuitively, infection is less likely to occur. Furthermore, IgG is not the only factor available to the foal for immediate defense against microbes, thus despite FPT, other components of the immune system may provide sufficient protection.
ACUTE PHASE RESPONSE
An acute phase protein is any protein whose blood concentration significantly increases during an inflammatory response.30 Collectively, acute phase proteins are responsible for many of the well-recognized reactions to microbial invasion, such as fever, anorexia, depression, and alterations in metabolism, hemodynamics, coagulation, and leukocyte activation. The liver is a key site of synthesis of the acute phase proteins, however some, such as ceruloplasm, complement components, and serum amyloid A, can also be produced extrahepatically.
Cytokines, principally tumor necrosis factor, interleukin 1β and interleukin 6, glucocorticoids, and growth factors stimulate and modulate gene expression and the transcription of the acute phase proteins. The serum concentrations of the major acute phase proteins—serum amyloid A (SAA) and C-reactive protein (CRP), and α-1-acid-glycoprotein (AGP)—can each increase as much as 1000-fold during the acute phase response.30 Interestingly, despite their intense synthesis during the acute phase reaction, the roles of each of these major proteins are still not entirely clear. SAA may be involved in cholesterol regulation, chemotaxis, and mediating anti-inflammatory events, such as down-regulation of fever, phagocytosis, and prostanoid synthesis. CRP can activate complement, induce phagocytosis, and stimulate cytokine and tissue factor expression. AGP is considered an anti-inflammatory agent as it reduces both complement- and phagocyte-mediated tissue injury and inhibits platelet aggregation.30 SAA concentrations have been determined by use of a latex agglutination assay in both healthy and ill foals.31 The expected SAA concentration in healthy Thoroughbred foals one to three days of age is <27 mg/liter, whereas foals with focal infection or septicemia have >100 mg SAA/liter. C-reactive protein concentrations have been established in healthy foals32 and adult horses with experimentally induced inflammation, however their utility in determining an inflammatory response in naturally-occurring diseases in foals has not be fully established.
The remaining acute phase proteins have widely diverse pathophysiologic effects. The complement system is represented by the acute phase synthesis of C3, C4, C9, Factor B, C1 inhibitor, and C4b-binding protein.30 If a host is immunologically naïve to a pathogen, and therefore does not have specific antibody, the alternative complement pathway is evoked, activating compounds that will induce bacteriolysis, provide components that are chemotactic for neutrophils, and enhance neutrophil opsonization of both microbes and damaged host cells. The importance of complement in host defense is exemplified by the finding that opsonization of E. coli and Actinobacillus are reduced by 50% in heat-treated serum, a process that destroys complement.33
Balanced activation of the coagulation and fibrinolytic systems by the acute phase response of factor VIII, fibrinogen, plasminogen, tissue plasminogen activator, plasminogen activator inhibitor, fibronectin, von Willebrand factor, and tissue factor leads to formation of intravascular and extravascular “clots” that serve to capture and contain infectious organisms and inflammatory debris and to provide a scaffold for tissue repair.34
SIRS, CARS, MARS, AND MODS
The ultimate goals of the innate immune system and the acute phase response are to contain infection, alarm the host to defend, and promote tissue repair. Whether these goals are achieved or defeated, the process of infection and defense against it may lead to systemic illness. In other words, even a successful campaign against microbial invasion can produce systemic disease. The host relies on a defense response from the innate immune system that is appropriate for the insult.18,35 If the innate immune system overzealously responds, the same components that are meant to protect the host may ironically be just as detrimental as or even more harmful to the host than the initial bacterial insult. It is the intensity of the invasion and the degree of balance in the host’s immune response that determine the final deposition of a microbial assault.
When the retort to infection results in an incongruous and exaggerated systemic inflammatory reaction, the clinical state is referred to as the Systemic Inflammatory Response Syndrome (SIRS).1 It is important to note that although this discussion of SIRS focuses on an infectious stimulus, SIRS can be initiated by noninfectious insults, such as severe trauma, hypothermia, hyperthermia, or intense hypoxemia. Sepsis and septicemia are SIRS induced by local and blood infection, respectively. To counteract the pro-inflammatory response and deter the state of SIRS, the host relies on anti-inflammatory opposition that includes production of cytokines (interleukins 4, 10, 11, 13), soluble cytokine receptors, receptor antagonists, prostaglandin E2, and corticosteroids.36
If there is over-recruitment of the anti-inflammatory processes, a state of anergy, increased susceptibility to infection, and inability to repair damaged tissues ensues. This scenario is referred to as Compensatory Anti-inflammatory Response Syndrome (CARS).1 In some circumstances, a Mixed Anti-inflammatory Response Syndrome (MARS) arises in which surges of both SIRS and CARS coexist.1 In the circle of equilibrium, if SIRS and CARS are ultimately appropriately balanced, then homeostasis resumes. Predominance of SIRS may culminate in adverse pathophysiologic events, such as disseminated intravascular coagulation (DIC), shock, organ failure, and death. In this later scenario, dissonance has occurred and the patient is defined as having Multiple Organ Dysfunction Syndrome (MODS) or the presence of organ dysfunction associated with acute illness in which homeostasis can not be restored without intervention.1
In summary, infection of the neonatal foal results from a combination of exposure to microbes and colonization of host tissue coupled with failure of the innate immune system to prevent microbial proliferation and invasion into deeper tissue. The outcome of infection is further determined by the appropriateness of the host’s defense response. With these facts in mind, when obtaining a history on neonatal foals, careful attention should be directed toward identifying potential exposures to microbes (in magnitude and virulence), as well as identifying factors that may affect immunocompetency of the patient. Box 5-1 summarizes historical events that have been associated with increased risk of sepsis in the neonatal foal.
Box 5.1 Historical Factors Associated with Increased Risk of Acquired Infection in the Perinatal Period
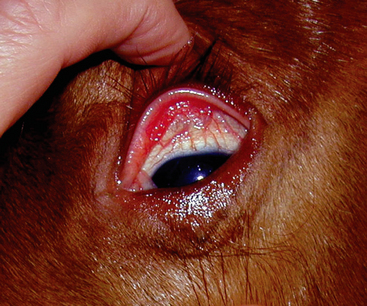
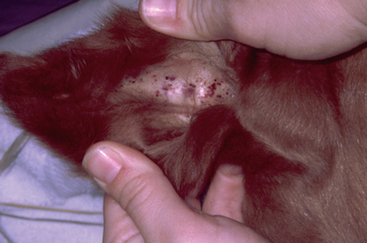
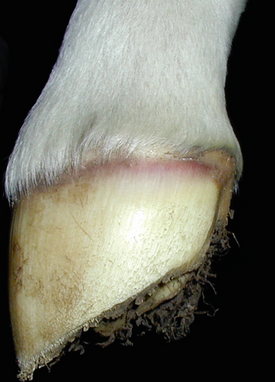
At the time of presentation to the Veterinary Teaching Hospital, Bugs Bunny was bright, alert, and responsive with normal vital signs. She appeared to have an adequate supply of milk. Her foal was recumbent, but able to stand with assistance. The foal’s rectal temperature was 102.9°F (39.3°C), heart rate was 110 beats/minute, and respirations were 30 breaths per minute. The foal weighed 53 kg. The sclerae were moderately to severely injected (Figure 5-2), numerous petechiae were present in the pinnae (Figure 5-3), and the coronets were hyperemic (Figure 5-4). Low-volume yellow diarrhea was passed.