3 Neonatal Immunology
Case 3-1 Foal with Failure of Passive Transfer
’03 Vulnerable, a bay colt weighing approximately 50 kg, (Figure 3-1) stood within one hour of birth and nursed vigorously from his dam within two hours. The farm veterinarian examined the colt at approximately six hours of age (the morning after foaling) and found no significant abnormalities. A repeat physical examination at 36 hours of age was also within normal limits. A blood sample was collected at the time of the second examination for evaluation of serum IgG concentration.
HISTORY AND PHYSICAL EXAMINATION
The equine neonate is immune competent but immune naïve at birth. Equine lymphocytes are capable of responding to antigenic stimulation as early as 80 to 100 days of gestation and can mount a humoral response to pathogens by 200 days of gestation.1,2 However, the fetus is protected from exposure to pathogens while in utero, and it is rare for a primary immune response to be elicited in a foal prior to birth. Maternal immunoglobulins are not transferred to the foal in utero because of the diffuse epitheliochorial placenta of the mare. The result is a foal that is born essentially agammaglobulinemic,3–5 not yet primed to mount a rapid anamnestic response, and profoundly susceptible to infection with environmental pathogens unless the foal ingests adequate amounts of colostrum, the first milk produced by its dam.6–9 Colostrum is widely recognized as the primary source of immunoglobulin for the equine neonate, but it also contains other immunologically important substances. Failure of the foal to ingest or absorb sufficient quantities of colostrum, primarily as defined by absorption of IgG, is termed failure of passive transfer (FPT). A foal with FPT is defined as a foal with a serum IgG concentration of <400 mg/dl at 24 hours of age. Partial FPT is defined as a foal with a serum IgG concentration of 400-800 mg/dl at 24 hours of age.
The reported incidence of complete or partial FPT in foals varies from 3% to 37.8%.6,10–17 This wide variation is often attributed to differences in management practices on individual farms. Indeed, implementation of management factors designed to decrease the incidence of FPT in foals resulted in a significant decrease in the number of affected foals on farms in the United Kingdom.16 However, in one survey of 158 Standardbred mares on a single consistently managed farm, the incidence of FPT varied over a five-year period from 4% to 14%. This suggests that management may not be the only important factor.18 Risk factors for FPT include month of foaling, adverse health events for mare and/or foal in the periparturient period, age of the dam, and colostral immunoglobulin content. A low colostral immunoglobulin concentration is associated with breed (Standardbred), age of dam (older mares) and total solar radiation.15,18,19
Poor-quality colostrum is difficult to identify based on appearance or available historical information; confirmation of this problem requires testing of the colostrum prior to ingestion by the foal. Increased solar radiation was associated with increased IgG concentration in colostrum and foal serum in a study by LeBlanc and colleagues,19 perhaps explaining the observation that FPT is more common in foals born between December and March in the northern hemisphere.18 Colostral IgG concentration was highest in mares three to ten years of age, and mean values were higher in Thoroughbred and Arabian mares than in Standardbred mares. When assessing colostral IgG concentration, it is also important to consider total quantity of colostrum produced. A mare with colostrum of lower IgG concentration may not be problematic if that mare produces a large total quantity of colostrum.
Production of colostrum with inadequate immunoglobulin content has been associated with ingestion of endophyte-contaminated fescue grass or hay. The causative agent of fescue toxicity is Neotyphodium coenophialum (formerly known as Acremonium coenophialum), a fungal endophyte that grows on stems, leaves, and seeds of tall fescue grass.20,21 In addition to agalactia, pregnant mares consuming contaminated fescue may experience thickened fetal membranes, premature placental separation with “red bag” delivery, retained placenta, prolonged gestation, and birth of weak foals with high perinatal mortality. The ergopeptine toxins responsible for most of the reproductive manifestations of fescue toxicity act as dopamine receptor agonists that inhibit prolactin secretion. This is compounded by decreased fetal progesterone secretion and increased serum estradiol concentrations. The net effect of decreased prolactin and progesterone and increased estradiol-17β is considered the cause of agalactia and insufficient mammary development in mares.
Foals may develop FPT even if they are known to have ingested adequate quantities of good-quality colostrum, presumably because of a failure to absorb ingested immunoglobulins. This is most often recognized in premature or dysmature foals, possibly as a result of immature gastrointestinal function, but may also occur in otherwise healthy and vigorous full-term foals. Glucocorticoids enhance the maturation of small-intestinal epithelial cells and hence their loss of absorptive capacity22,23 leading to speculation that endogenous corticosteroids released secondary to stress at the time of parturition may impair immunoglobulin absorption in foals.3,24 However, administration of adrenocorticotrophic hormone to stimulate endogenous corticosteroid release failed to affect absorption in experimental foals,25 and stress has not been a consistent historic finding in foals with FPT due to presumptive impaired immunoglobulin absorption.13
Foals with FPT as a sole problem do not exhibit any recognizable clinical signs. Abnormalities on physical examination are reflective of other disease processes. Therefore, unless a farm has a screening program to identify foals with FPT during the first few days of life, the problem is not recognized unless the foal becomes sick. FPT is a major risk factor for neonatal septicemia, omphalophlebitis, septic arthritis, and other infectious diseases and should be suspected in foals exhibiting clinical signs consistent with any of these disease conditions.6–8
PATHOGENESIS
Colostrum is produced in the mammary gland during the last weeks of gestation. As part of this process, immunoglobulin is concentrated from the mare’s blood into colostrum in the mammary gland. While the predominant immunoglobulins in equine colostrum are IgG (1500-5000 mg/dl) and IgG(T) (500-2500 mg/dl), IgM (100-350 mg/dl) and IgA (500-1500 mg/dl) are also present.26 At the time of parturition, colostral IgG concentration may exceed 9000 mg/dl in some mares.27 The immunoglobulin concentration in milk decreases to negligible levels within 12 hours when mares are actively suckled by a healthy foal.
While immunoglobulin is the only component of colostrum that is routinely measured in veterinary medicine, colostrum also contains a wide range of other soluble and cellular substances that may have a positive impact on neonatal immunity and gastrointestinal maturity. These include cytokines,28 growth factors,29 hormones,29 enzymes,30 lymphocytes, macrophages, neutrophils, and epithelial cells.27 The cellular components of colostrum likely play a role in local gastrointestinal immunity in the foal. Maternal cells may be found in mesenteric lymph nodes, blood, lungs, liver, and spleen of the neonate after ingestion of colostrum, suggesting they may play a role in systemic immunity as well.
Normal foals begin suckling colostrum within one to two hours of birth, and maternal antibodies are detectable in the blood of the foal within six hours. Specialized cells distributed throughout the small intestine nonselectively absorb macromolecules by pinocytosis.5 Absorbed proteins pass through the intercellular space and lacteals into the systemic circulation via the lymph. Maximal absorptive efficiency occurs immediately after birth, declining to only 22% efficiency at 3 hours after birth and less than 1% by 20 hours.31,32 This rapid decline in absorptive efficiency is due to rapid turnover of absorptive cells in the gastrointestinal mucosa and their replacement with more mature cells that are unable to nonselectively take up macromolecules.5,12 The decline in absorption of colostral proteins parallels a transient proteinuria that peaks by 6-12 hours of age and declines by 24-36 hours of age.5,33 Neonatal proteinuria most likely reflects absorption and excretion of low–molecular-weight milk proteins.
The half-life for disappearance of maternal antibodies from the foal’s circulation varies between 20 and 30 days.34–36 Antibody concentrations decline as a result of normal catabolism and gradual dilution in an increasing plasma volume as the foal grows.5 There is also evidence for transfer of functional IgG into the gastrointestinal tract, which may account for partial clearance from the blood and enhanced gastrointestinal immunity.37,38 Most maternal antibodies are present in only negligible concentrations by 6 months of age, although antibodies to some infectious agents have been detectable for up to 12 months after birth. The duration of detection of specific antibodies most likely depends on the quantity of specific antibodies absorbed and the sensitivity of the assay used. As passive antibody levels wane, autogenous antibody production increases with autogenous IgG first detectable at approximately two to four weeks of age.35 The result is a nadir in serum immunoglobulin concentrations in the colostrum-fed foal at approximately one to two months of age followed by gradually increasing concentrations until adult levels are reached at approximately 5 to 10 months of age.3,39 Serum immunoglobulin concentrations are similar in colostrum-fed and colostrum-deprived foals by three to four months of age.
DIAGNOSIS
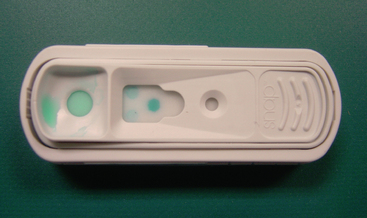
Ideally, all foals should be examined by a veterinarian and assessed for adequacy of passive transfer of immunoglobulin when they are 18 to 48 hours old. Foals considered to be at high risk for FPT and/or sepsis may be tested as early as 6 to 12 hours of age.40 Serum IgG concentration should be assessed in all foals less than one month of age presenting for any type of medical problem. Foals should have a serum IgG concentration of >800 mg/dl to provide optimal passive immunity. A variety of rapid screening test kits are available for stallside use to detect FPT in foals.41–47 Important factors to be considered in selecting a screening test for a specific farm include overall accuracy, time necessary to perform the test, simplicity of the testing procedure, and cost. Quantitation of total serum protein by refractometry, although technically simple and cheap to perform, is relatively inaccurate as an indicator of serum IgG concentrations in foals.47 However, there is a close correlation between serum IgG concentrations and total serum globulin concentrations in clinically healthy foals with no hematological abnormality.16 Zinc sulfate and glutaraldehyde coagulation tests are relatively accurate, inexpensive, and results are available within an hour.45,48 Many veterinarians and farm managers prefer to use commercial kits based on either glutaraldehyde coagulation or enzyme-linked immunosorbent assays44,45 (Figure 3-2). In a recent comparison of the glutaraldehyde coagulation and the enzyme-linked immunosorbent assay (SNAP, Idexx Inc., Portland, ME), both tests were appropriate screening tests for FPT because they had high sensitivity and negative predictive value, meaning that foals with adequate IgG are identified correctly. The enzyme-linked immunosorbent assay was better than the glutaraldehyde coagulation in specificity and positive predictive value, meaning that foals with FPT were identified correctly. Immunoturbidometric testing of foal blood for serum IgG concentration is being increasingly used and appears to perform adequately in the field.49
To Treat or Not to Treat
Foals that are intentionally colostrum-deprived rapidly succumb to septicemia, even when housed in a clean environment with good management practices.9 Several field studies have confirmed that FPT is a risk factor for disease in the first few months of a foal’s life.15,18 However, Baldwin and colleagues failed to show any statistical link between serum IgG concentration and prevalence of illness, severity of illness, or survival of foals.10,50 Hence, there is some debate regarding the significance of FPT or partial FPT in vigorous, healthy foals on well-managed farms that are known to have received some colostrum but have a serum IgG concentration of <800 mg/dl.51 Not all foals with FPT transfer will develop septicemia, but they are at higher risk than foals with adequate IgG. Foals from mares that have previously lost foals due to infection should also be considered high-risk foals.
TREATMENT
Normal equine plasma with high concentrations of IgG is commercially available from several sources. The veterinarian should choose a USDA-licensed product to assure appropriate quality control in production. Donor horses should be routinely screened for equine infectious diseases and for major alloantigen or alloantibody incompatibilities. Ideally, donor horses should be negative for the Aa and Qa alloantigens to prevent sensitization of recipients to future transfusions. Donors should also be negative for all known equine alloantibodies to diminish the likelihood of adverse reaction in the recipient. For maximum benefit to the foal, donor horses should be vaccinated against common equine pathogens and contain appropriate concentrations of equine disease-specific antibodies. Some manufacturers also vaccinate their donor horses with lipid A in order to have a high level of anti-endotoxin antibodies.
If the concentration of IgG in the donor plasma is known, the quantity of plasma to be transfused can be estimated. In normal foals, a dose of 200 mg IgG/kg body weight raised the serum IgG concentration by 450 mg/dl; a dose of 400 mg/kg of IgG increased the serum IgG by 575 mg/dl.51 However, other studies suggest that there is no direct correlation between the quantity of IgG transfused and the increase in the foal’s IgG concentration.16 Septic foals often require a greater total volume of plasma to increase serum IgG concentrations by the same amount. Administering 1 liter of normal equine plasma with average quantities of IgG will typically increase the serum IgG concentration of a 45 kg foal by 200 to 300 mg/dl.53 In order to achieve a final serum IgG concentration of >800 mg/dl in a foal with complete FPT (initial IgG <200 mg/dl), a total of 2 to 4 liters of normal equine plasma may be required.
To administer plasma, an intravenous catheter should be placed aseptically in one jugular vein. Frozen plasma should be thawed and warmed slowly to room temperature using a warm water bath. Microwave thawing or thawing with very high temperatures should be avoided because of the possibility of denaturing important plasma proteins. All blood products that are administered intravenously should be administered through an appropriate in-line blood filter to remove fibrin clumps and other debris (Figure 3-3).
The foal’s serum IgG concentration should be determined approximately 12 to 24 hours after administration of plasma to confirm that the desired increase has been achieved. The delay in assessing posttransfusion concentrations is necessary to allow time for distribution of the transfused immunoglobulin into vascular and extravascular spaces. In addition to redistribution into extravascular spaces, transfused immunoglobulin is rapidly catabolized or used in immune reactions. Healthy foals transfused with plasma at one day of age experienced a 30% decrease in serum IgG concentrations by seven days of age.54 This decline might be even more precipitous in septic foals with increased vascular permeability and increased immune demands.
PREVENTION
Farm managers should be encouraged to maintain a clean foaling environment during the periparturient period. Thorough cleaning and disinfection of foaling stalls between mares, replacing soiled bedding immediately after parturition, and washing the dam’s perineal and mammary areas before the foal stands may decrease the ingestion of potentially pathogenic bacteria from the environment as the foal initiates environmental suckling in search of the mare’s udder. Although most foals presenting for treatment of septic diseases have FPT,6,7,16 partial FPT is not always associated with increased prevalence of illness or death.10,16,18 Madigan has suggested that even minimal ingestion of colostrum may be associated with decreased disease because of a hastening of gut closure and prevention of absorption of bacteria,55 however further studies suggest that feeding does not hasten gut closure.56 Ensuring that the foal receives some colostrum immediately after birth by nasogastric tube or bottle feeding has been recommended to ensure that colostrum is present in the gastrointestinal tract prior to any exposure to bacteria.55
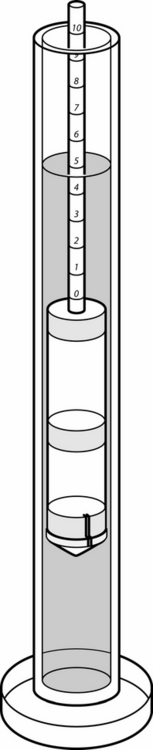
If foaling is attended, a sample of presuckle colostrum may be obtained to quantitate colostral IgG. Good colostrum tends to be sticky, yellow, and thick, but these subjective criteria are unreliable in assessing quality. Several rapid screening tests are available for stallside assessment of colostrum. One widely used method for estimating colostral quality is determination of colostral-specific gravity using a “colostrometer” (Equine Colostrometer, Lane Manufacturing Company, Denver, CO) (Figure 3-4). Specific gravity correlates directly with the IgG concentration of the colostrum. Approximately 75% of foals that ingest colostrum with a specific gravity of <1.060 will have serum IgG concentrations of <400 mg/dl; when specific gravity is >1.060, foals usually attain serum IgG concentrations of >500 mg/dl.57 Sugar refractometry using a handheld refractometer (Brix 0-50% sugar refractometer) is also widely used for assessment of colostral quality.58,59 A Brix % sugar reading of 20% to 30% correlates with adequate colostral quality and a reading of >30% indicates very good-quality colostrum55 (Figure 3-5). A commercial kit based on glutaraldehyde coagulation (Gamma-Check-C, Veterinary Dynamics, Inc., San Luis Obispo, CA) is available for stallside screening of immunoglobulin concentrations in mare colostrum.60
Studies on the induction of lactation show that normal milk production with high IgG concentration can be produced by barren mares. These results may suggest an alternative source of colostrum for freezing.61
If the risk for FPT is recognized during the first 8 to 12 hours of life and equine colostrum is not available, bovine colostrum62–64 or a commercial colostrum substitute65–69 may be administered to the foal. Bovine colostral antibodies are absorbed by foals, but the half-life of these antibodies is considerably shorter than for equine antibodies. Approximately 2 to 4 liters of bovine colostrum should be administered; many foals develop transient mild diarrhea. Foals given only bovine colostrum as a source of passive antibody and raised under experimental conditions had a high incidence of respiratory disease, emphasizing the desirability of using equine colostrum whenever possible.
Lyophilized equine IgG (Lyphomune, BIOQUAL, Inc., Rockville, MD) is available as an equine colostral substitute. A minimum of 50 to 70 g of IgG is recommended for treatment of the average 45-kg foal that receives no colostrum, but in one study this dose failed to increase serum IgG concentration to >450 mg/dl in colostrum-deprived foals.69 A concentrated equine serum product (Seramune, Sera, Inc., Shawnee Mission, KS) is available for use in foals with FPT. However, it failed to increase serum IgG concentrations in colostrum-deprived foals to adequate levels, probably because of the relatively low total IgG dose that was administered.65 Because the product is not clearly labeled as to IgG concentration, it is difficult to formulate specific recommendations regarding the exact quantity to be administered. If the product contains 25 g of IgG per 300-ml bottle as stated by Vivrette,65 approximately three bottles may be required to increase the serum IgG concentration of a 45-kg colostrum deprived foal to >400 mg/dl. Whereas colostrum substitutes derived from lypholized serum IgG may better used as colostrum supplements, those made from freeze-dried equine colostral IgG may have better efficacy.70
If there are no other available sources of immunoglobulin for a foal, oral administration of equine plasma or serum may be considered. This is an expensive source of oral immunoglobulin, and approximately 2 to 4 liters would be required to treat a colostrum-deprived 45-kg foal.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree