2 High-Risk Pregnancy
Case 2-1 Placentitis in the Peripartum Mare
Vital Connection, a 13-year-old Thoroughbred mare, presented on January 29, 2004, with a primary complaint of premature-onset lactation. Her only cover for this pregnancy was March 25, 2003. Mammary development had begun two weeks prior to presentation, at which time Regumate (altrenogest, Intervet, Inc., Millsboro, DE) was administered at 10 cc twice daily by mouth (Figure 2-1). She stabilized until five days prior to presentation when her udder suddenly increased in size again. She was treated with intramuscular estrogen in addition to the oral altrenogest. She again stabilized until the day prior to presentation when she showed signs of early labor or mild colic and had wax present at both teats. Her reproductive history included five previous gestations. The first three gestations resulted in parturition occurring approximately “two weeks early” and nonsurviving foals dying at three to four days of age. No further specifics were available, although she may have been in Kentucky during the “Mare Reproductive Loss Syndrome” (MRLS) outbreak. The last two gestations produced normal healthy foals with an average gestation length of 333 days. For the last three gestations, including this one, she had received 10 cc Regumate PO daily until two weeks before her anticipated foaling date.
HISTORY AND PHYSICAL EXAMINATION
Prepartum disorders in the mare are usually readily recognized, but disorders of the fetus and placenta can be more subtle and difficult to determine. Problems resulting in premature mammary development generally fall into one of three categories: placentitis, twins, and incorrect breeding date. The first step in determining which might be the case is acquisition of a thorough history of the mare. Of particular interest is any history of previous abnormal foals. However, the history taking should also include questions regarding transportation, establishment of an accurate breeding date (sometimes more difficult than one would suspect), and any pertinent medical history (including diagnostic testing performed for the pregnancy such as culture, endometrial biopsy, and cytology, and any rectal and ultrasound examination results). Additionally, information regarding possible ingestion of endophyte-infected fescue or exposure to potential infectious causes of abortion should be obtained.1,2 A complete vaccination and deworming history is requisite, as is a complete history of any medications and supplements administered during pregnancy.
DIAGNOSTICS
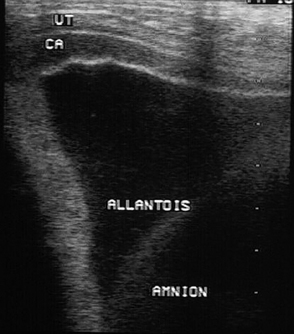
Ultrasonographic evaluation of the uterus and conceptus per rectum can provide valuable information, particularly regarding placental thickness if placentitis is a concern (Figure 2-2). Fetal fluids can be evaluated and fetal size can be estimated from the size of the eye later in gestation.3 In our hospital we choose not to perform vaginal examinations or speculum examinations as we have seen an association between these exams and the subsequent development of placentitis and, unless placentitis is recognized with ultrasonographic evaluation per rectum and culture is desirable, these types of examinations are generally not necessary.
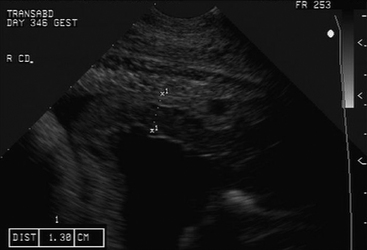
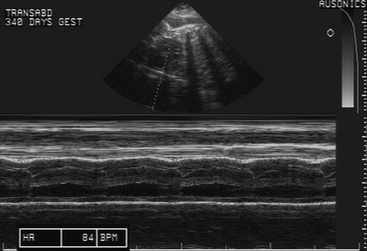
Following examination per rectum, transabdominal ultrasonographic evaluation of the uterus and conceptus is performed4 (Figure 2-3). The sonogram is performed through the acoustic window present from the udder to the xiphoid ventrally and laterally to the skinfolds of the flank (Figure 2-4). Imaging of the fetus usually requires a low-frequency (3.5 MHz) probe, while examination of the placenta and endometrium usually requires a higher frequency (7.5 MHz) probe (Figure 2-5). The utility of this type of examination lies in its repeatability and low risk to the dam and fetus. Sequential examinations over time will allow the clinician to follow the pregnancy and identify changes as they occur.
A biophysical profile of the fetus consisting of heart rate, fetal movement, placental thickening, and aminiotic/allantoic fluid volume can be generated from this examination in the late term fetus, and viability can be readily determined4,5 (Table 2-1). The presence or absence of twins is also readily determined in the late pregnant mare in this manner. Uteroplacental thickening (>13 mm), placental separation (anechoic spaces between the chorion and uterus), and sudden increases in fetal fluid turbidity may be associated with placentitis.4
Table 2-1 Late Gestation Biophysical Profile of Fetal Placental Unit Generated by Transabdominal Ultrasound Examination
| Parameters | Normal |
|---|---|
| Fetal heart rate | 70–90 beats/minute |
| Fetal activity | Less than 10 minutes of inactivity |
| Placental thickness | 7–13mm |
| Amniotic fluid depth | 0–8cm |
| Allantoic fluid depth | 0–13cm |
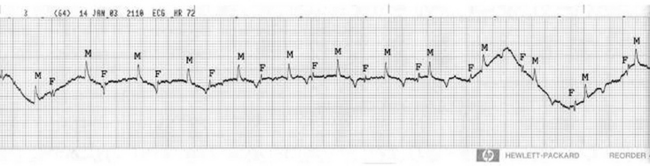
A companion to transabdominal ultrasonography is evaluation of the fetal electrocardiogram (ECG).4,6,7 Fetal ECGs can be measured continuously using telemetry or can be obtained using more conventional techniques several times throughout the day. Electrodes are placed on the skin of the mare in locations aimed at maximizing the magnitude of the fetal ECG but, because the fetus frequently changes position, multiple sites may be needed in any 24-hour period. We begin with an electrode placed dorsally in the area of the sacral prominence with two electrodes placed bilaterally in a transverse plane in the region of the flank (Figure 2-6). The fetal ECG maximal amplitude is low, usually 0.05 to 0.1 mV, and can be lost in artifact or background noise, so it is common to move electrodes to a new position to maximize the appearance of the fetal ECG. The normal fetal heart rate during the last months of gestation ranges from 65 to 115 bpm, a fairly wide distribution (Figure 2-7). The range of heart rate of an individual fetus can be quite narrow, however. Bradycardia in the fetus is an adaptation to in utero stress, and is usually thought to be hypoxia. By slowing the heart rate, the fetus prolongs exposure of fetal blood to maternal blood, increasing the time for equilibration of dissolved gas across the placenta and improving the oxygen content of the fetal blood. The fetus also alters the distribution of its cardiac output in response to hypoxia, centralizing blood distribution.8,9 Tachycardia in the fetus can be associated with fetal movement, and brief periods of tachycardia should occur in the fetus in any 24-hour period. Persistent tachycardia is a sign of fetal distress and represents more severe fetal compromise than bradycardia.10

Fig. 2-6 Placement of ventral-lateral fetal ECG electrode.
Reprinted with permission from Wilkins PA: Monitoring the pregnant mare in the ICU, Clin Tech in Equine Pract 2:212-219, 2003.
PATHOGENESIS
Placentitis is a common cause of late-term abortion in mares and perhaps the most common cause of mares presenting with clinical signs of precocious udder development, premature lactation, cervical softening, and vaginal discharge.11 The cause is generally considered to be ascending infection that enters the uterus via the cervix, although hematogenous spread of some bacterial and viral agents (EHV-1 and EVA in particular) is possible. Mares with poor perineal conformation, abnormal cervical anatomy (sometimes secondary to previous birth trauma), a history of vaginal/cervical examination performed late in pregnancy, or a history of being placed in dorsal recumbency while pregnant, are at increased risk of placentitis, although for many there is no identified underlying cause.
Common bacteria that have been isolated in equine placentitis/abortions include Streptococcus equi (subspecies zooepidemicu), Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumoniae, and nocardioform species.12
Fetal loss and premature delivery secondary to placentitis are not fully understood. Recent studies, however, suggest that infection of the chorioallantois results in increased expression of inflammatory mediators that, in addition to other local effects, alters myometrial contractility.12,13,14 Combined, these observations suggest that fetal loss can occur due to a compromised fetus, increased myometrial contractility, or both.
TREATMENT
The approach to management of the high-risk pregnancy is dictated to some degree by the exact cause for concern, but for many mares therapy is similar.15,16 Many high-risk mares present with placentitis, primarily caused by ascending bacterial or fungal infections originating in the region of the cervix. These infections can cause in utero sepsis or compromise the fetus by local elucidation of inflammatory mediators or altered placental function. Premature udder development and vaginal discharge are common clinical signs. Treatment consists of administration of broad-spectrum antimicrobial agents and nonsteroidal anti-inflammatory drugs (Table 2-2). Trimethoprimsulfa (TMS) drugs appear to cross the placental/uterine barrier. Studies by Sertich and Vaala have demonstrated increased concentration of these agents in the fetal fluids when compared to less detectable levels of penicillin and gentamicin.17 Other research by Murchie and colleagues using microdialysis showed that gentamicin and penicillin were found in the allantoic fluid after administration to mares. The elimination of these drugs from the allantoic fluid was slower than that from the mare’s serum probably due to lack of mechanisms to eliminate drugs in this compartment.18 If culture and sensitivity results are available, directed therapy should be instituted toward the specific organism isolated.
Table 2-2 Drugs Used to Treat Mares with High-Risk Pregnancy
| Drug | Dose/Frequency/Route | Reason |
|---|---|---|
| Trimethoprim sulfonamide | 25mg/kg BID PO | Antimicrobial |
| Flunixin meglumine | 0.25mg/kg TID PO/IV | Anti-inflammatory |
| Altrenogest | 0.44mg/kg SID PO | Tocolytic |
| Isoxuprine | 0.4–0.6mg/kg BID PO | Tocolytic |
| Clenbuterol | 0.8 g/kg PRN PO | Tocolytic |
| Pentoxifylline | 4–6gm/500kg BID PO | Anti-inflammatory |
| Vitamin E | 1,000–10,000IU/day PO | Antioxidant |
SID = once daily, BID = twice daily, TID = three times daily, PRN = as needed, PO = by mouth, IV = intravenously.
Nonsteroidal anti-inflammatory agents, such as flunixin meglumine, are used in an effort to combat alterations in prostaglandin balance that may be associated with infection and inflammation. Although the efficacy of these agents is best when administered prior to the development of clinical signs, to date no detrimental effects have been reported in the fetus or dam when chronically used at low doses in well-hydrated patients. In the Murchie study, there was an attempt to measure flunixin meglumine levels in the allantoic fluid postadministration to pregnant mares. It was determined that flunixin meglumine was not measurable due to the method of microdialysis.18 Pentoxifylline has been used for its rheologic effect, potentially improving blood flow within the placenta, and also for its general anti-inflammatory effect.19–21
Tocolytic agents and agents that promote uterine quiescence have been used and include altrenogest (Regumate, Intervet, Inc.) isoxuprine, and clenbuterol.22–26 Although the use of altrenogest or progesterone in late gestation has been challenged, one study found that progestin supplementation helped to prevent prostaglandin-induced abortion in early gestation.27 Though the mechanism of action is unclear, some feel that progestins have an anti-prostaglandin effect by interfering with prostaglandin and oxytocin receptors, thus reducing myometrial activity.28 The efficacy of isoxuprine as a tocolytic in the horse is unproven, and bioavailability of orally administered isoxuprine appears to be highly variable. Clenbuterol may be indicated during management of dystocia in preparation for assisted delivery or caesarian section because it has been shown to decrease uterine tone for up to 120 minutes when administered intraveneously.29 Unfortunately, the intravenous form of clenbuterol is not currently available in the United States. In a more recent study by Palmer and colleagues, 29 pregnant pony mares were treated daily in late gestation with intravenous clenbuterol; the treatment did not delay normal partuition.30 The long-term use of clenbuterol is inadvisable due to receptor population changes associated with chronic use and its unknown effects on the fetus at this time.
Three additional strategies can be used in managing high-risk pregnancy patients. Where there is evidence of placental dysfunction, with or without signs of fetal distress, we provide intranasal oxygen supplementation to the mare in the hope of improving oxygen delivery to the fetus. Intranasal oxygen insufflation of 10 to 15 L/min has been shown to significantly increase both PaO2 and percent oxygen saturation of hemoglobin (% O2 sat).31 Because of the placental vessel arrangement of the horse, improvement of these two arterial blood gas parameters should result in improved oxygen delivery to the fetus.32,33 Blood gas transport is largely independent of diffusion distance in the equine placenta, particularly in late gestation, and is more dependent on blood flow. Information from other species cannot be extrapolated to the equine placenta because of its diffuse epitheliochorial nature and the arrangement of the maternal and fetal blood vessels within the microcotyledons.34,35 Umbilical venous PO2 is 50 to 54 mmHg in the horse fetus, compared to 30 to 34 mmHg in the sheep, while the maternal uterine vein to umbilical vein PO2 difference is near zero. Also un-like the sheep, the umbilical venous PO2 values decrease 5 to 10 mmHg in response to maternal hypoxemia and increase in response to maternal hyperoxia.36,37
Vitamin E (tocopherol) is administered orally to some high-risk mares as an antioxidant for both the placental/uterine inflammation and as a neuroprotectant strategy for the fetus. Administration of large doses of vitamin E prior to traumatic brain injury improves neurologic outcome in experimental models and has been examined as possible prophylaxis for human neonatal encephalopathy (NE).38–40 Extrapolation of that information to the compromised equine fetus suggests that increased antioxidant concentrations in the fetus may mitigate some of the consequences of uterine and/or birth hypoxia, but no evidence is available to date demonstrating that protection occurs, or that vitamin E accumulates in the fetus in response to supplementation of the mare. Recent evidence suggests that large (>5,000 IU/day) vitamin E doses do not increase maternal vitamin E concentration more than smaller doses (1,000 IU/day).41 In that study, no increase in plasma vitamin E concentration was seen in the newborn foal at any dose; however, vitamin E accumulation in tissues was not directly examined.
Finally, many high-risk mares are anorexic or held off feed because of their medical condition. These mares are at particularly large risk for fetal loss due to their lack of feed intake, which alters prostaglandin metabolism.42 Therefore, intravenous dextrose, 2.5% to 5% dextrose in 0.45% saline or water (5% dextrose), at fluid rates providing 1 to 2 mg/kg/min dextrose, should be administered to these patients.
Few of the strategies described above are specifically aimed at the fetus, but rather in maintaining the pregnancy. Recently, evidence has appeared that prenatal ACTH administration may be beneficial in advancing the maturity of the fetus.43 In a compromised pregnancy where clinical signs of early delivery do not regress with treatment, this therapy, or exogenous corticosteroid therapy, may be considered to increase the chances of fetal survival. However, potential risk for loss of the fetus may well outweigh any advantage provided by fetal ACTH administration.
Perhaps the most important aspect of managing high-risk pregnancy mares is frequent observation and development of a plan. Mares should be observed at least hourly for evidence of early-stage labor and should be under constant video surveillance if possible. Depending on the primary problem, the team managing the mare should develop a plan for handling the parturition once labor begins and for fetal resuscitation following delivery. Any equipment that might be needed should be readily available stallside. A call sheet, listing contact numbers for all involved, should be posted on or near the stall. The plan should include a decision as to how to handle a complicated dystocia with permission for general anesthesia and cesarian section obtained prior to the event so that time is not wasted.44,45 It is important to ask the owner at the outset which is more important, the mare or the foal, as the answer may dictate the direction of the decision tree once labor begins.
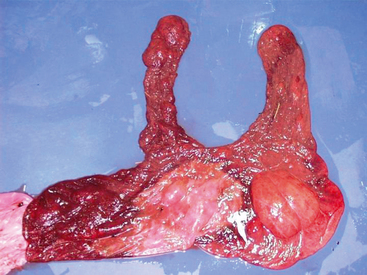
The fetal membranes were intact and malodorous despite being passed in a timely manner. There was a 15 by 30 cm well-demarcated tan area of discoloration at the base of the horns associated with a thick coffee-colored exudate. Approximately 80% of the chorion was discolored with the tip of the pregnant horn particularly discolored. Samples were obtained for culture and histopathology. The allanotoic surface was unremarkable. The amnion had prominent vasculature and was slightly edematous with opaque red-tan watery exudate present on the chorionic surface (Figure 2-8).
PLACENTAL EXAMINATION
The placenta of each pregnancy should be examined grossly to insure that the entire placenta has been expelled. There are serious complications, such as endotoxemia and laminitis, for the mare if pieces are retained. For mares with suspected placentitis, placental examination may provide clues to the cause of the inciting problem. Knowledge of the normal placental anatomy and normal artifacts that can be found in the placenta is helpful in distinguishing normal from abnormal.46
Structures of the placental unit include the umbilicus, the chorioallantois, the amnion, and the hippomanes. The amnionic umbilicus consists of two arteries, a vein, and the urachus. These vessels loosely spiral along the length of the umbilicus. The length of the umbilicus varies with different pregnancies but averages about 55 cm in the thoroughbred fetus.47 Hippomanes are amorphous tan to brown oval-shaped masses that are found in the allantoic cavity of most mares. Their significance is unknown.46
On presentation, the chorioallantois is usually inverted from its normal orientation within the mare with the allantoic side on the outside, unlike the chorionic placental presentation seen in Vital Connection. The allantoic surface should be glistening and whitish with prominent vessels. If one orients the placenta so that the chorionic surface is on the outside, then a red velvetlike surface is apparent. The color can vary in places because of uneven expulsion of blood from the placenta during parturition.46
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree