11 Gastrointestinal Disease
Case 11-1 Colic in the Newborn Foal
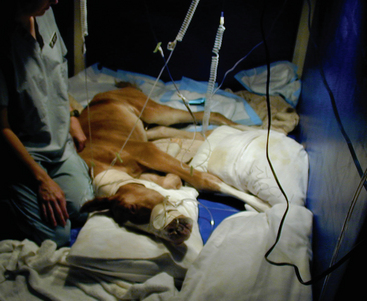
At the time of presentation, ’03 RockN’Roll was approximately 26 hours old. The foal was quiet, but tracked the mare well and was interested in nursing. The abdomen was slightly distended, and meconium staining was present on the tail and perineum. Shortly after admission, the foal was observed to raise his tailhead and strain (Figure 11-1). A small amount of urine was passed. The rectal temperature was 100° F, heart rate was 88 beats/minute, and respirations were 40 breaths/minute. Borborygmi were audible on both sides of the abdomen. A nasogastric tube was passed, and no reflux was obtained. A digital rectal examination confirmed the presence of firm meconium at the pelvic inlet. Palpation through the abdominal wall revealed at least a 12-cm segment of bowel in the left caudoventral abdomen that was approximately 3 cm in diameter and contained firm ingesta. Intermittent straining, as well as flank biting, continued. The foal then became recumbent and rolled up onto his back. There were no other significant physical findings. The major problems identified were straining to defecate, mild abdominal distension, acute abdominal pain (colic), and firm feces at the pelvic inlet.

Fig. 11-1 Straining to defecate. ’03 RockN’Roll at the time of presentation to the Veterinary Teaching Hospital at approximately 26 hours of age. The foal is exhibiting signs of straining to defecate: raised tail head, slightly arched back, hindlimbs slightly under the trunk. Note the meconium staining on the tail and perineum. The white tape on the foal’s back was used as a landmark to serially measure the circumference of the foal’s abdomen. Compare this foal’s stance to the stance in Figure 11-4.
DIAGNOSTIC APPROACH TO COLIC IN NEONATAL FOALS
According to the results of the National Animal Health Monitoring System report on colic in horses in the United States in 1998 and 1999, the incidence of colic in foals less than six months of age was approximately 18 times less than the incidence in mature horses.1 However, when considering aliments affecting foals, gastrointestinal tract problems and infection were most commonly reported.2,3 Because some etiologies of colic are unique in the neonatal period, special consideration must be given to the evaluation of abdominal pain in this age group.
In some respects, the diagnostic approach to colic in neonatal foals is similar to that used in mature horses: rarely will any single fact be useful in determining the exact etiology. However, careful and simultaneous inspection of multiple historical, physical, and diagnostic findings may be formative in determining the anatomical location of the lesion (stomach, small intestine, large intestine, peritoneal cavity), the etiologic category (congenital, nonstrangulating obstruction, strangulating obstruction, inflammatory, or other), or even possibly the specific diagnosis (Table 11-1). In obtaining a history, particular attention should be given to the farm history, use of medications (especially analgesics), risk factors for septicemia or failure of passive transfer, and problems with other foals on the farm.
As some differentials are strictly age-dependent (see Table 11-1), knowing the age of onset of clinical signs is important. In the neonatal period, commonly reported causes of abdominal pain are meconium impaction, small-intestinal volvulus, enteritis or colitis, uroperitoneum, intussusception, gastric ulcers, and ileus secondary to prematurity, septicemia, or neonatal encephalopathy.4–8 Clinical signs of lethal white syndrome, meconium impaction, and uroperitoneum most commonly manifest in the first 12 to 24 hours, 12 to 96 hours, and 48 to 96 hours of life, respectively. However, if uroperitoneum is the result of urachal or urinary bladder infection, clinical signs may be delayed until 7 to 14 days of age. Although the potential spaces created by congenital or traumatic umbilical, inguinal, and diaphragmatic hernias may be present since birth, incarceration of bowel into these spaces may occur at any age, if at all. Enteritis, colitis, intussusception of small intestine, small-intestinal volvulus, and clinically significant gastric ulcers may develop at any age.
The breed and sex of the foal may provide supportive evidence for certain differential diagnoses. Lethal white syndrome is most commonly reported in all-white to almost-all-white offspring of overo cross overo Paint Horses. In one study of 168 horses with congenital umbilical hernias, the incidence was two times greater in fillies compared to colts, and Thoroughbreds were twice as likely to have an umbilical hernia compared to Standardbreds.9 In this later study, incarceration of bowel into the umbilical hernia was not reported. Scrotal hernias most commonly occur in Standardbred and Tennessee Walking Horse colts,8 and fecaliths are frequently reported in American Miniature foals.6,10 In one study, meconium impactions occurred twice as often in colts compared to fillies.11 Although the incidence of uroperitoneum is often quoted to occur more commonly in colts, in one recent study, the incidence was approximately equal among the sexes.12 Additional historic information that should be carefully scrutinized is the general health of the dam, the foal’s gestational age, the foaling history and general perinatal health, such as ingestion of colostrum, age at passage of meconium, nursing frequency (see Chapter 1 for review of normal perinatal health), and farm history of potentially infectious causes of enteritis or colitis (Salmonella, rotavirus, Clostridium). Most foals will have passed meconium within 9 to 12 hours of life; however, the gastrocolonic reflex stimulated by ingestion of colostrum frequently initiates earlier passage of meconium. Evacuation of meconium may be delayed (meconium retention) as the result of ileus secondary to another primary nongastrointestinal disease, such as septicemia or neonatal encephalopathy. In these cases, although passage of meconium may be slower than expected, the delay in passage may not be accompanied by clinical signs of abdominal pain. Passage of “milk feces” or yellow pasty feces does not necessarily indicate that all meconium has been removed from the colon.
CLINICAL SIGNS
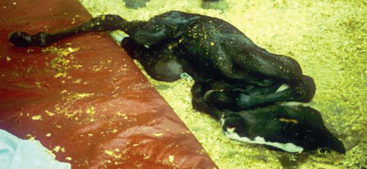
Clinical signs of abdominal pain in foals can be highly variable, and the intensity of the signs is not necessarily indicative of the etiology. It is important to note that foals with inflammatory lesions of the intestinal tract or those suffering from general functional ileus secondary to systemic disease can act as violently painful as foals with obstructive or strangulating lesions of bowel. However, in general, foals suffering from uroperitoneum and gastric ulcers have less intense abdominal pain than do foals with inflammatory or obstructive lesions. Repeatedly thrashing or rolling from side to side is generally accepted as a highly representative clinical sign of abdominal pain. However, early signs of abdominal pain in neonatal foals may only manifest as reduced frequency of nursing and prolonged recumbency. Other subtle but significant signs of abdominal pain in foals include general restlessness, especially in recumbency, and/or frequent adjustment of recumbent positions (Figure 11-2). Often recumbent foals with abdominal pain will stretch their limbs, twist their head or neck, roll into dorsal recumbency (Figure 11-3), and make frequent attempts or strain to either defecate and/or urinate. In the standing foal, signs that are classically associated with straining to defecate include frequent tail swishing, a “water spout” tail, and a “camped under” leg stance with a dorsiflexed back (Figure 11-1). In contrast, a flat or ventroflexed back with the hindlimbs stretched backward and the tail held up is associated with urination (Figure 11-4). Other signs of abdominal pain include lip curling, flank biting or watching, pawing at the ground, and kicking at the abdomen. It is important to recognize that often critically ill foals that are stuporus will not overtly demonstrate classic signs of abdominal pain, despite the presence of serious gastrointestinal disease.

Fig. 11-4 Stance to urinate. This foal is demonstrating the typical stance seen with urination: tail straight up, back flat, hind limbs slightly stretched back. Compare this foal’s stance to the stance in Figure 11-1.
The colicky neonatal foal should receive a thorough general physical examination with careful attention given to identification of clinical signs or physical evidence of sepsis (see Chapter 5). Sepsis may be directly associated with the primary etiology of the abdominal pain or it may develop secondary to bacterial translocation if the integrity of the gastrointestinal mucosa is compromised. The presence of fever, depression, petechiae, injection, synovitis, uveitis, or diarrhea may be cardinal clues that the abdominal pain has an inflammatory etiology. Although the presence of diarrhea may be an important sign of enterocolitis, intense abdominal pain frequently precedes the onset of diarrhea. Tachycardia and/or tachypnea are expected findings despite etiology. Persistent tachycardia (heart rate >120 beats per minute) has been suggested to be more commonly associated with surgical disorders of the abdomen, compared to medical etiologies.13 Gross abdominal distension will develop with gas or fluid accumulation in the bowel or abdomen. Severe abdominal distension with intense pain that elicits high-pitched “pings” with percussion is consistent with gas-distended large intestine or cecum. Repeated measurement of the abdominal circumference may be helpful in more objectively determining the course of progression of abdominal distension. Typically, the absence of intestinal sounds is not helpful in determining etiology; however, increased borborygmi may be associated with enteritis or colitis. If diarrhea is present with signs of abdominal pain in a foal, additional fecal diagnostics may be indicated (see Case 11-3).
Unlike mature horses, transabdominal wall palpation can be performed in the neonate and may identify gastric distension, hepatomegaly, thickened or distended bowel, meconium, and the urinary bladder. The ventral abdomen and inguinal rings should be carefully examined for evidence of herniation. Ballottement or succussion of the abdomen may verify a fluid wave compatible with excess fluid in the peritoneal cavity. With adequate restraint or sedation, a careful digital rectal examination may identify meconium or ingesta in the pelvic inlet. A nasogastric tube should be passed in all foals with clinical signs of abdominal pain. Compared to mature horses, successful retrieval of gastrointestinal reflux can be frustrating in the neonate, even in the presence of proximal intestinal disease. Frequently, nasogastric tubes that are designed for neonatal enteral feeding are of insufficient size for gastric decompression. With this in mind, the largest-bore tube that can be comfortably passed should be used, and persistent attempts should be made to obtain gastric reflux, either by suction or priming the tube with water.
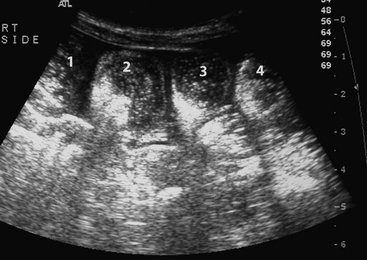
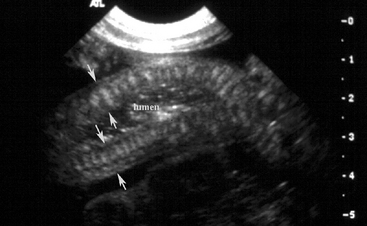
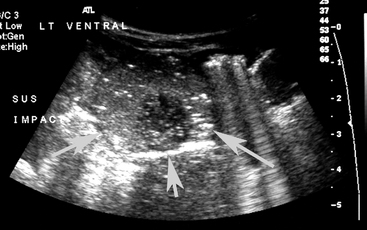
Blood was obtained for a blood culture, and a 16 g polyurethane catheter was placed in the jugular vein. A transabdominal ultrasound examination was performed using a 7-4 MHz probe and revealed ingesta of variable echogenicity, surrounded by a gas pattern, in several sections of bowel in the left caudal dependent abdomen (Figure 11-5). The diameter of the bowel in this location was approximately 2 cm. There were no other significant findings on the transabdominal ultrasound examination. Based on the history, physical findings, and transabdominal ultrasound examination, meconium impaction was suspected. Based on the presence of a significant left shift and partial failure of passive transfer, the potential for secondary sepsis was increased.
CLINICAL PATHOLOGY
Many clinicians are hesitant to perform an abdominocentesis on a neonatal foal as the risk for complications such as inadvertent enterocentesis or omental prolapse occur more commonly in foals undergoing an abdominocentesis, as compared to adult horses. Many clinicians perform a transabdominal ultrasound examination first to assess potential risks of performing an abdominocentesis versus the likelihood of successfully obtaining peritoneal fluid. If an ultrasound examination reveals widespread and/or grossly distended loops of intestine, and/or free peritoneal fluid is difficult to identify, the chances of an uncomplicated and successful abdominocentesis are reduced. An abdominocentesis may be completed on the standing foal or with the foal in lateral recumbency. In either position, proper restraint is imperative and is best facilitated by light sedation (Table 11-2).
More commonly, use of a teat cannula results in inadvertent prolapse of omentum through the abdominocentesis site upon collection of peritoneal fluid or removal of the cannula. If this happens, the omentum should be sharply transected with a sterile blade at the abdominal wall and tucked back into the abdominal cavity with another sterile teat cannula.
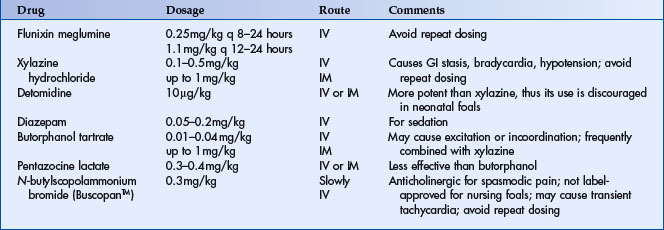
Normal peritoneal fluid should be light yellow in color and clear. The normal mean nucleated cell count in the peritoneal fluid of healthy foals was reported to be 450 cells/μl (range 60 to 1,400 cells/μl) for 17 foals that were 13 to 134 days of age14 and 1,400 cell/μl (±1077) for 32 Thoroughbred foals that were 14 to 75 days of age.15 Both of these reference ranges for nucleated cell counts are lower than means reported for healthy adult horses. The mean protein concentration by biochemical biuret determination and refractive index in foals was reported to be 1.2 g/dl and 1.6 g/dl, respectively.14 The interpretation of peritoneal fluid nucleated cell count and protein concentration is similar to adult horses with abdominal pain and does not necessarily definitively distinguish etiology or medical from surgical causes of colic. Sanguinous fluid can be present with either severe enterocolitis or strangulating lesions of bowel. Special attention should be given to the cytologic examination. The presence of degenerative neutrophils or bacteria is worrisome (Figure 11-6) and is indicative of loss of the mucosal integrity, bowel rupture, or primary sepsis in the peritoneal cavity. In one study, 78% of colicky foals with peritoneal fluid protein concentrations >2.5 g/dl that subsequently underwent an exploratory laparotomy were euthanized.8 When uroperitoneum is suspected, the creatinine concentration of both peritoneal fluid and serum should be simultaneously assessed. A peritoneal fluid creatinine to serum creatinine ratio >2 is supportive evidence of uroperitoneum. When determining peritoneal fluid creatinine concentration on an automated chemistry analyzer, it is important to use plasma or serum methodology on the abdominal fluid. If urine methodology is used to determine the creatinine concentration on a peritoneal fluid sample, erroneously increased creatinine concentration may lead to an incorrect diagnosis of uroperitoneum.
DIAGNOSTIC IMAGING
Unlike mature horses, abdominal radiography can provide useful information in a colicky neonatal foal. Grid, rare-earth screens and sufficient mAs (5 to 28) and kVp (80 to 120) should be used.16 Radiographs may be taken with the foal either standing or in lateral recumbency; however, keep in mind that fluid-gas interfaces are easier to detect in the standing foal, when the radiographic beam is horizontal or perpendicular to the dorsoventral plane of the gas/fluid interface in the abdomen. Both right and left lateral views should be obtained as some structures will be more obvious, depending upon which side of the abdomen is closer to the film. Ventrodorsal views may only be possible in small foals or those that are stuporus, sedated, or anesthetized. This view will optimize identification of the pylorus, the descending duodenum, the base of the cecum, the left colon, and the transverse colon.17 Some degree of gas is normally visible in the stomach, small intestine, cecum, and small colon.
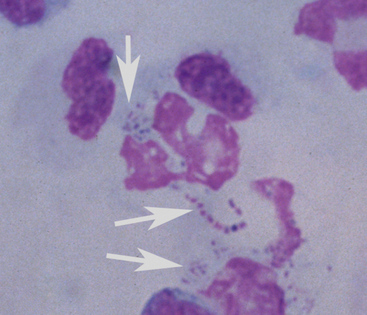
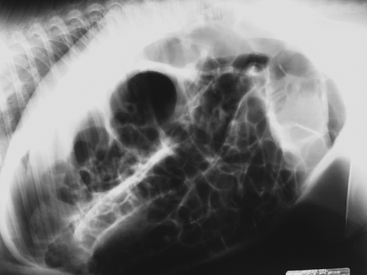
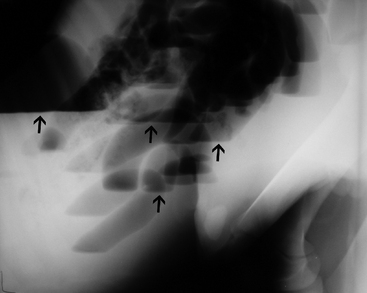
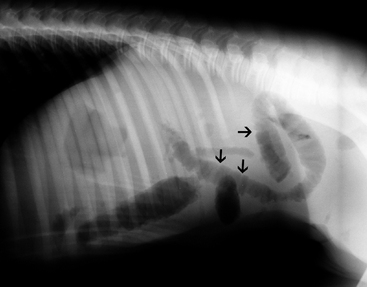
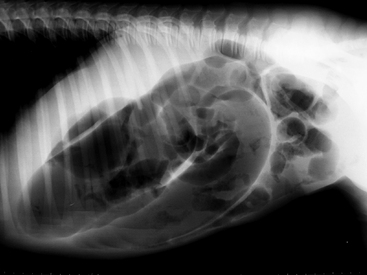
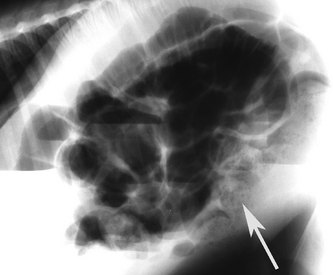
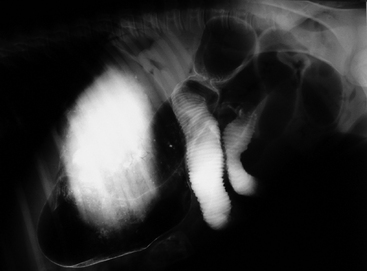
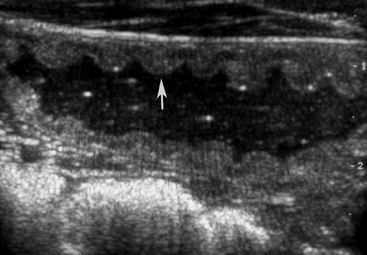
In general, plain film abdominal radiology is more likely to provide information on the anatomic location of the problem than information directly leading to the exact etiology.8,16,17 For example, gas distension of the small intestine is a nonspecific finding that may occur with enteritis, functional obstruction (ileus), or mechanical obstruction (Figure 11-7). However, if small-intestinal distension is accompanied by “hairpin” or “U-shaped” turns of the bowel (Figure 11-8) or multiple, uneven intraluminal gas-fluid interfaces (Figure 11-9), it is more likely associated with mechanical obstruction, though these findings can also occur with enteritis or functional ileus. Thickened walls of small intestine may appear uneven or “corrugated” when caused by enteritis (Figure 11-10). Severe generalized gas distension of large intestine is more commonly associated with mechanical obstruction than with inflammatory lesions of large colon (Figure 11-11). Meconium frequently appears as granular contents in the ascending or descending colon (Figure 11-12). Pneumoperitoneum suggestive of bowel rupture should be suspected if there is a gas cap in the dorsal aspect of the abdominal cavity, if the serosal surfaces of bowel are enhanced, or if visualization of the renal silhouettes is improved. Iatrogenic pneumoperitoneum should be considered if an abdominocentesis was performed prior to abdominal radiography.
Finally, abdominal radiography obtained on foals with necrotizing enterocolitis may demonstrate the pathognomonic sign of pneumatosis intestinalis, or intramural air. Radiographic signs of pneumatosis intestinalis are spectacular and include localized cystic collections that appear as radiolucent “bubbles” in the bowel wall, or diffuse linear strips or flat oval-shaped areas of radiolucency in the bowel wall flanked by the radiopaque serosa and mucosa, when the bowel wall is viewed end-on.18
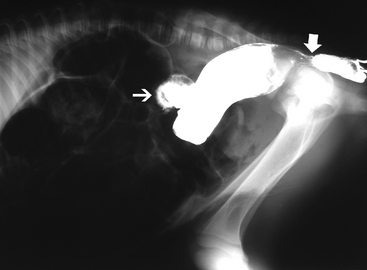
Contrast radiography also has some specific indications in neonatal foals. An upper gastrointestinal contrast study can be used to document delayed gastric emptying of older foals with suspected pyloric outflow obstruction.16 Foals less than two weeks of age ideally should be fasted for four hours, and foals consuming solid feeds should be fasted for 12 hours prior to contrast radiography.17 Barium is administered by gravity flow via a nasogastric tube (5 ml/kg as a 30% weight/volume solution), and abdominal radiographs are obtained every 30 minutes. If barium remains in the stomach for longer than two hours, delayed gastric emptying should be suspected but does not necessarily distinguish between mechanical or functional obstruction. Barium should normally reach the cecum and transverse colon by two hours and three hours, respectively, in 10- to 12-day-old foals.16,17 Barium transit time to the transverse colon is five to eight hours in foals one to two months of age. In addition to documenting transit time, stenosis or abnormal bowel wall may be highlighted by the contrast (Figure 11-13). Lower-intestinal contrast studies (i.e., barium enema) have been reported to have 100% sensitivity and 100% specificity for identifying mechanical obstruction (meconium impaction, atresia coli) of the transverse colon or small colon in foals less than 30 days of age (Figure 11-14).19 The foal should be restrained or lightly sedated and placed in lateral recumbency. A 24-french Foley catheter is placed into the rectum and the bulb gradually inflated. By gravity flow, administer up to 20 ml/kg of 30% weight/volume barium.
Two-dimensional transabdominal ultrasonography is a valuable diagnostic tool in the colicky neonatal foal. Scanning techniques for the foal abdomen are extensively reviewed elsewhere,20 though a brief review follows here. Standard linear array 4 to 7 MHz transducers are sufficient for visualization of most intra-abdominal structures in the neonatal foal; however a curvilinear transducer will optimize image quality. The foal may be scanned in either lateral recumbency or standing; however keep in mind that fluid-filled, thickened, or enlarged structures may descend to the dependent portion of the abdomen and could be overlooked if only the nondependent side of a recumbent foal is scanned. Furthermore, gas in nondependent bowel, especially gas in large colon, may preclude examination of deeper structures in laterally recumbent foals. Visualization of intra-abdominal structures is facilitated by clipping the abdominal hair; however thorough wetting of the hair with water or alcohol, in addition to acoustic coupling gel, may be sufficient. In the colicky neonate, transabdominal ultrasonography can be used to identify fluid-distended structures (i.e. stomach, small intestine, large intestine, urinary bladder), gastric or intestinal wall thickness, abnormal intestinal contents (i.e. meconium, fecaliths, phytobezoars, or trichophytobezoars), peritoneal fluid, and to determine intestinal motility.
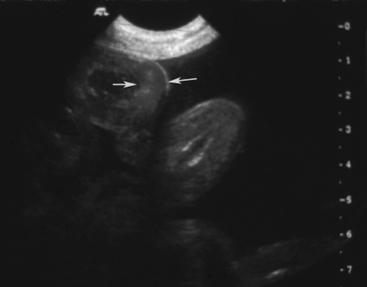
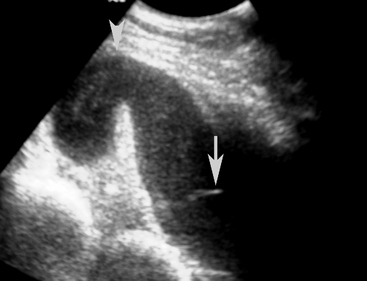
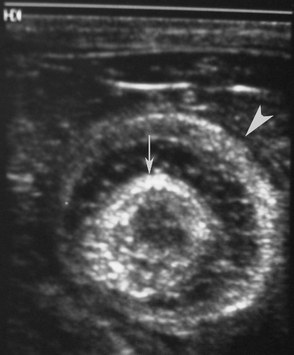
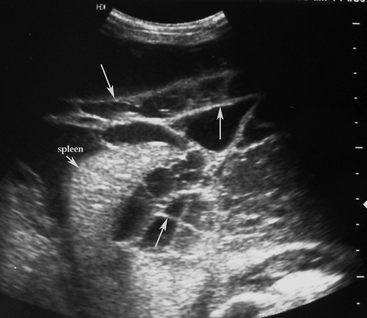
Normal gastric wall thickness in foals should be less than 7 mm, whereas intestinal wall thickness is 3 to 4 mm. Foals with gastritis or gastric ulcers may have a thickened or irregular gastric wall. Likewise, foals with enteritis or colitis will frequently have diffusely increased small and/or large intestine wall thickness (Figure 11-15). This feature alone does not definitively identify enteritis, as edematous bowel that develops as the result of strangulation (volvulus or intussusception) will also appear regionally thickened (Figure 11-16), with distended fluid-filled intestine proximally. The presence of gas echoes in the intestinal wall, a thickened hypoechoic wall that appears “wavy,” or the presence of sloughed mucosa in the lumen are more consistent with a diagnosis of enteritis and should be considered as distinguishing features from strangulating lesions (Figure 11-17). Foals with enteritis or colitis may demonstrate either hypermotile or hypomotile bowel, whereas functional ileus and mechanically obstructed intestine more often will appear hypomotile. Neonatal foals with mechanical obstruction of small intestine may have hairpin or U-shaped turns of the small intestine when viewed in long axis (Figure 11-18). The unique regional presence of a double intestinal wall or what appears to look like a “bull’s-eye” of multiple concentric rings in a short axis view of small intestine is consistent with an intussusception (Figure 11-19), which most commonly is seen in the dependent portion of the abdomen.
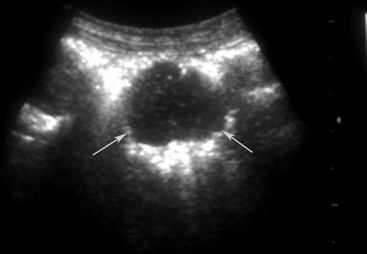
Meconium may appear hyperechoic, hypoechoic, or as a mixture of echogenicities in hypomotile intestine. Fluid- or gas-distended intestine may be present proximal to the obstruction (Figures 11-20 and 11-21). When present in the small colon, retained or impacted meconium often appears as a row of “balls” and is most easily identified in the dependent portion of the left caudal abdomen in the standing foal. It may be traceable dorsal to the urinary bladder and often is surrounded by a thin layer of hyperechoic gas in sacculated intestine (small colon). Meconium in large colon typically is more amorphous. Extensive gas in the large colon frequently hinders visualization of other intra-abdominal structures and can be associated with mechanical obstruction, though it may also occur with acute colitis. The sonographic appearance of abdominal abscesses is variable, but in the neonatal foal, intra-abdominal abscesses are often associated with umbilical remnants, previously devitalized bowel, or mesenteric lymph nodes. Finally, the presence of excessive anechoic fluid may be indicative of uroperitoneum (see Chapter 12), though anechoic transudate may also develop as a result of enteritis, colitis, or strangulation. Hyperechoic densities within peritoneal fluid may represent fibrin strands, leukocytes, or adhesions (Figure 11-22). The presence of gas echoes free within the peritoneal fluid is indicative of a ruptured viscus.
Gastroscopy may be accomplished with a 1-meter endoscope in a neonatal foal and is covered elsewhere in more detail later in this chapter (see Case 11-2). For most neonatal foals, suckling can be allowed prior to gastroscopy, but solid-feed consumption should be withheld for 6 to 10 hours.21 Caution should be exercised in the interpretation of the clinical significance of gastric ulcers in foals, as up to 50% of young asymptomatic foals have gastric ulcers along the margo plicatus of the greater curvature of the stomach, and gastric ulcers may develop secondary to another primary gastrointestinal disorder.22,23 However, multiple deeper ulcers, bleeding ulcers, ulcers along the lesser curvature or in the glandular mucosa, or those accompanied by clinical signs that are consistent with gastric ulceration should be considered clinically significant.
DIFFERENTIAL DIAGNOSIS
Most information on colic in the neonate is obtained from referral institutions or, more often, from foals undergoing exploratory celiotomy for acute abdominal pain; therefore the data may not precisely reflect the true incidence of each reported etiology. The only published study on 20 foals less than two weeks of age with acute abdominal pain reported that an exploratory celiotomy revealed functional ileus (45%), meconium impaction (25%), large-colon displacement (15%), small intestine displaced around the base of the cecum (10%), ruptured gastric ulcer, and small colon obstructed by the ovarian ligament.7 Other retrospective studies of abdominal surgery in the foal include foals three to six months of age and thus it was difficult to ascertain the etiology of abdominal pain exclusive to the neonatal period. In 53 foals that underwent an exploratory celiotomy from birth to three months of age, meconium impaction, uroperitoneum, enteritis, small-intestinal strangulation (herniation with incarceration, small-intestinal volvulus, and intussusception), and enteritis accounted for 80% of the total cases.4 In a study that reviewed 83 foals up to six months of age, the most commonly reported diseases identified at surgery were small-intestinal volvulus, meconium impaction, and intussusception.8 These reports underscore the difficulty in definitively identifying the cause of abdominal pain prior to exploratory celiotomy in neonatal foals, as clearly some of these cases, such as enteritis and functional ileus, would not be considered to be predominantly surgical diseases. Furthermore, it is noteworthy that if the etiology of the abdominal pain cannot be ascertained in the violently painful patient, an exploratory celiotomy may be indicated solely as a diagnostic tool. The purpose of this section is to provide a brief review of the conditions most commonly reported in the neonatal foal. Table 11-1 provides a more comprehensive list of reported causes of acute abdominal pain in the young foal.
Meconium Impaction
Meconium is the sticky caramelized feces of the newborn foal that comprises intestinal secretions, swallowed amniotic fluid, and cellular debris. In one study of 30 newborn foals, it was reported that the total weight of meconium is equal to 1% of the foal’s body weight.24 Most foals will start to evacuate meconium shortly after the first ingestion of colostrum, which acts both as a laxative and stimulator of the gastrocolonic reflex. The majority of the meconium is evacuated within the first 12 hours of birth and is replaced by “milk” feces, which are pasty and yellow in appearance. However, concurrent disease such as neonatal asphyxia, prematurity, septicemia, and encephalopathy may delay passage. Meconium impaction implies failure to evacuate sufficient quantities of meconium with subsequent development of signs of colonic obstruction: pain, straining to defecate, and abdominal distension secondary to accumulation of gas in bowel proximal to the impacted meconium.
It has been suggested that meconium impaction is more likely to occur in colts11 and in foals greater than 340 days of gestational age.25 To the author’s knowledge, prophylactic use of an enema at birth has not been shown to prevent meconium retention or impaction. Foals with meconium impaction are disinterested in nursing, strain to defecate, and typically stand with a slightly arched back and frequently “swish” their tail (Figure 11-1). Digital examination may detect meconium within the rectum. Colic, abdominal distension, tachypnea, general restlessness, and tachycardia are frequently reported clinical signs.11 Intestinal borborygmi are usually present11 and are not a reliable sign of obstruction. Meconium can usually be identified by either plain or contrast abdominal radiography or ultrasonography (see “Diagnostic Approach to Colic in Neonatal Foals” section above). In more severe obstructions, excessive amounts of gas may accumulate in the large colon, proximal to the obstruction. Rupture of the urinary bladder may occur in foals that strain excessively from meconium impaction.11 Furthermore, extensive mural damage lends to bacterial translocation and secondary septicemia.
Lethal White Syndrome
Other causes of acute abdominal pain that closely mimic meconium impaction and primarily manifest during the first 24 to 48 hours of life are the rarely reported cases of ileocolonic aganglionosis, atresia coli, and atresia recti. Ileocolonic aganglionosis, or “lethal white” syndrome, is a fatal autosomal recessive disorder principally of overo cross overo Paint Horses that is caused by a point mutation in amino acid 118 in endothelin receptor B.26 The endothelin receptor is critical for the proper development and migration of cells from the neural crest that ultimately form melanocytes in the skin as well as neurons in the intestinal tract. Thus, foals that are homozygous for the mutation are essentially all white (though some small areas of pigmentation can occur) and develop signs of functional ileus in the first few hours of life (Figure 11-23). Color patterns in the dam and sire that have the highest incidence of heterozygote carriers of the mutation are frame overo, highly white calico overo, and frame blend overo horses.26 However, the heterozygote mutation has been occasionally rarely detected in other white-patterned Paints and “nonPaint” white-patterned bloodlines. It has not been detected in solid-colored breeding-stock Paint Horses without white, but heterozygote adult solid-colored Miniature Horses of Paint lineage and white-patterned horses of breeds other than the Paint Horse have been rarely identified.26
Lethal white foals typically are born “normal” in appearance and behavior, with the exception of the mostly white coat. Clinical signs of abdominal pain usually develop in the first few hours of life, after ingestion of colostrum, and progressively develop gross abdominal distension. Evidence of passage of meconium is often missing, though some meconium may be passed. The only way to definitively identify a lethal white foal is by DNA testing for the mutation or histopathologic demonstration of insufficient intestinal ganglia. Unfortunately, it can take weeks to obtain the results of DNA testing and hours to days to obtain the results of intestinal biopsies. Diagnosis is often initially presumptive, based on signalment, heterozygote parents, lack of response to symptomatic treatment, and rule out of other causes by additional diagnostics or surgical exploratory. Affected foals and heterozygote adults can be identified by submitting plucked hair with intact root bulbs from the mane (preferred sample) to the Veterinary Genetics Laboratory at the University of California, Davis, available at www.vgl.ucdavis.edu/service/horse/coatcolor.html. It should be noted that not all white foals of Paint lineage are lethal whites and when tested, these unaffected, rare, all-white Paint Horses are not homozygous for the mutation.
Other Differentials
Atresia coli, recti, or ani are rarely reported causes of colic in the newborn foal that may mimic meconium impaction, based on age of onset and signs of obstructive disease.27 It may be possible to identify atresia recti on visual or digital examination of the rectum or by protoscopy. Retrograde contrast barium enema usually identifies an abrupt obstruction (see Figure 11-14), but definitive identification may require an exploratory celiotomy. Successful surgical repair has been described; however, other congenital abnormalities have been simultaneously reported and should be ruled out prior to consideration of surgery. The heritability of these defects is not fully known.27
Fecaliths are hard concretions of ingesta (Figure 11-24) that may also contain undigested material, such as hair. They most commonly occur in the American Miniature breed and have been reported in foals as young as 19 days of age.6,10 Affected foals typically present with progressive unresponsive abdominal pain that is accompanied by gross abdominal distension. As fecaliths obstruct the small colon or rectal lumen, abdominal radiographs typically demonstrate gas distension of the large colon. Successful treatment almost inevitably involves a celiotomy with a small colon enterotomy.
Strangulating lesions of the small intestine that require surgery occur more frequently in neonatal foals, as compared to strangulating lesions of the large intestine. Volvulus of the small intestine is the most commonly reported strangulating lesion in foals less than three months of age.8 Factors leading to development of these lesions are undetermined, but alterations in motility may contribute. Intussusceptions are most commonly reported in three- to five-week-old foals2 and may be acute or chronic and intermittent. Small-intestinal intussusceptions are most frequent, but ileocecal and cecocolic intussusceptions have been reported in young foals.28 Most inguinal and umbilical hernias in foals resolve spontaneously without incarceration of intestine.8,29–32 In a large retrospective study of 147 foals with umbilical hernias, only 13 required surgical repair.29 Only 4 of the 13 foals that needed surgery had incarcerated bowel in the umbilical hernia. Although the hernias were present since birth, and strangulation may rarely occur shortly after birth, the majority of foals with strangulating umbilical hernias were not neonates, but most often, affected foals were presented in the first six months of life.31 The most common presenting complaint in foals with an umbilical hernia with incarcerated bowel was an acute firm enlargement of the hernia that was sensitive upon palpation. Only 30% of foals with strangulated umbilical hernias presented with signs of abdominal pain.
In separate retrospective reports, inguinal hernias in neonatal foals that resulted in incarceration of small intestine that required surgical correction were exclusively found in newborn foals as a result of rents in the vaginal tunic (direct inguinal hernia).30,32 Onset of clinical signs was typically within 48 hours of birth. Diagnosis should be suspected by the presence of an irreducible mass in the scrotal sac, edema in the scrotum and prepuce, and colic. Diagnosis of either an umbilical or scrotal hernia with incarceration can usually be confirmed by palpation and ultrasonography. It has been suggested that elective herniorrhagy should be considered if an umbilical hernia has been present in foals greater than six months of age or if the defect is larger than 10 cm.31
Diaphragmatic hernias are rare in foals and can result from failure of fusion of its embryonic components or from traumatic rupture of the diaphragm in utero, at birth, or after birth.33 Affected foals can remain asymptomatic for prolonged periods of time, but the presenting complaint typically is acute abdominal pain. The intensity and onset of clinical signs is related to the amount of intestine that has herniated into the thoracic cavity and whether the herniated bowel is simply displaced or strangulated.33 In addition to abdominal pain, tachypnea and/or dyspnea may be present. The diagnosis can be confirmed by either radiographic or ultrasonographic evidence of an incongruous diaphragmatic line, the presence of gas-fluid interfaces indicative of intestine in the thoracic cavity, and/or pleural effusion.
Enterocolitis, gastric ulceration, and uroperitoneum are commonly reported causes of abdominal pain in the neonate and are described separately later in this chapter and in Chapter 12. Functional ileus secondary to prematurity, sepsis, neonatal asphyxia, neonatal encephalopathy, electrolyte abnormalities, concurrent gastrointestinal disease, hypoxic or ischemia bowel, overfeeding, use of milk replacer, botulism, and many other concurrent diseases may induce significant abdominal pain in the neonatal foal. If these underlying conditions are present, careful consideration to additional diagnostics should be used to rule out more serious gastrointestinal disorders (see “Diagnostic Approach to Colic in Neonatal Foals” section above). Simple solutions, such as reducing or eliminating enteral feeding, changing the source of milk, or correction of electrolyte derangements and other underlying disorders should be tried first. However, it may be necessary to consider an exploratory celiotomy for definitive diagnosis and for therapeutic decompression of bowel before initiation of prokinetic therapy. Cecal impaction, large-colon volvulus, large-colon displacement, intestinal infarction, and ileal impaction are considered to be rare disorders of the neonatal foal.
To address the increased risk of sepsis, 1 liter of plasma was given intravenously and the foal was started on K penicillin (22,000 units/kg IV q 6 hours) and amikacin (21 mg/kg IV q 24 hours). Four hours after presentation, the blood glucose concentration decreased to 80 mg/dl and thus the LRS was supplemented with 2.5% glucose. The foal remained quiet and alert for the next several hours and was intermittently observed to strain. A small amount of meconium was passed six hours after admission. Approximately 10 hours after admission, the foal was observed to roll into dorsal recumbency. An abdominocentesis was performed using an 18-gauge needle. The peritoneal fluid was light yellow in color and had a nucleated cell count of 5,900/μl and a protein concentration of 2.6 g/dl. Cytologic examination revealed 95% nondegenerative neutrophils with occasional Dohle bodies, and 5% mononuclear cells. Microbes were not observed. The foal’s TPR remained within normal limits until 12 hours after presentation, when the rectal temperature was 100.5° F, the heart rate was 176 beats/minute, and respiration was 24 breaths/minute.
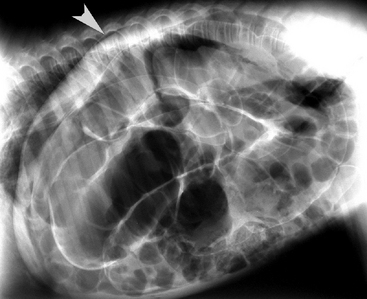
A digital rectal examination revealed continued presence of ingesta at the pelvic inlet. Abdominal radiographs were obtained (Figure 11-25) and demonstrated generalized gas distension in the large colon with granular-appearing contents in the lumen. The serosal margin detail was increased, and free gas was noted in the peritoneal cavity. The radiographic findings were consistent with obstruction of the large colon with pneumoperitoneum. The tachycardia and radiographic findings prompted the decision to perform an exploratory celiotomy. An extensive and firm meconium impaction was confirmed in the small colon. Despite aggressive attempts to dislodge the impaction by injection of the impaction with carboxymethylcellulose, accompanied by external mural massage of the small colon, a small colon enterotomy was necessary to remove the impaction. Upon further exploration of the peritoneal cavity, a small full-thickness tear of the transverse colon wall was identified with focal adjacent fecal contamination. With this revelation, the foal was euthanized under anesthesia. A necropsy examination was not performed.
TREATMENT
Medical therapy for meconium impaction includes judicious use of analgesics, intravenous polyionic isotonic fluids, oral laxative therapy, and enemas. Foals with meconium impactions are expected to exhibit some degree of pain. Judicious use of analgesics (Table 11-2) is required to balance the necessity to provide relief from pain and the ability to appropriately assess the patient’s progress. Mineral oil (4 to 8 ounces administered via a nasogastric tube) is used for its lubricating effect. Milk of magnesia (1 to 2 ounces) provides an osmotic laxative effect, but should be used sparingly as it may be dehydrating. The detergent dioctyl sodium sulfosuccinate can be quite irritating, and it should be avoided in both oral and rectal therapy.8 Castor oil therapy has been described,34 but can provoke violent abdominal pain in the foal.
Enemas are a mainstay of treatment for small-colon meconium impactions. Warm-water liquid detergent (i.e., Palmolive®) enemas (½ teaspoon liquid detergent to 500 ml water) are purportedly gentle to the rectal mucosa and effective. Commercial phosphate enemas (i.e., Fleet®) can also be used, but repeated administration may increase the risk of phosphate toxicity. Recently, acetylcysteine retention enemas have been reported to be a highly successful treatment for meconium impactions in foals.11 It is hypothesized that the acetylcysteine cleaves disulphide bonds in the mucoprotein molecules in meconium, decreasing its overall tenacity.
A 4% acetylcysteine solution, pH 7.6, is made by adding 20 g of baking soda and 8 g of acetylcysteine to 200 ml of water. A 30-french Foley catheter with a 30 ml bulb is inserted approximately 2.5 to 5 cm into the rectum and, the bulb is slowly inflated to occlude the rectum. One hundred to 200 ml of the 4% acetylcysteine solution is administered by gravity flow and retained for 30 to 45 minutes. The acetylcysteine retention enema was effective in eliminating 78% of meconium impactions within 12 hours.11 If needed, the acetylcysteine therapy can be repeated in 12 hours, and was repeated up to three times in some cases before resolution of the impaction. Occasionally, repeated use of an enema generates significant rectal and small colon mucosal irritation that sustains signs of straining despite effective removal of the impaction. This continued straining may confound determination of successful treatment.
Surgical intervention should be considered if medical therapy is unsuccessful.35,36
OUTCOME
The prognosis for foals with a meconium impaction is generally considered good to excellent with short-term survival reported to be 100%11 and long-term survival, following either medical or surgical treatment, reported to be 80% to 94%.35,36 Most meconium impactions will resolve with medical intervention. At the University of California, Davis, from 1987 to 2002, 41 out of 44 foals (93%) with meconium impactions were successfully treated medically with acetylcysteine enemas.11 About 40% of these former cases required more than one acetylcysteine enema, and in about 20% of cases it took more than 12 hours for the impaction to resolve. Once hospitalized, ’03 RockN’Roll received one acetylcysteine enema and oral laxative and intravenous fluid therapy over 12 hours. Prior to surgery, the foal exhibited only mild to moderate signs of intermittent pain (straining to defecate with recumbency and rare dorsal recumbency) and his abdominal size did not significantly increase. The discovery of pneumoperitoneum before celiotomy was worrisome. However, because an abdominocentesis had been performed several hours prior to taking the abdominal radiograph, it was speculated that the pneumoperitoneum was iatrogenic from introduction of room air through the abdominocentesis needle into the peritoneal cavity. The subsequent detection of a ruptured transverse colon during surgery was an unexpected complication. Spontaneous rupture of the colon as a sequela to meconium impaction must indeed be rare, as there are no reports of its occurrence in the American literature. It remains unclear as to when the colon rupture occurred.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree