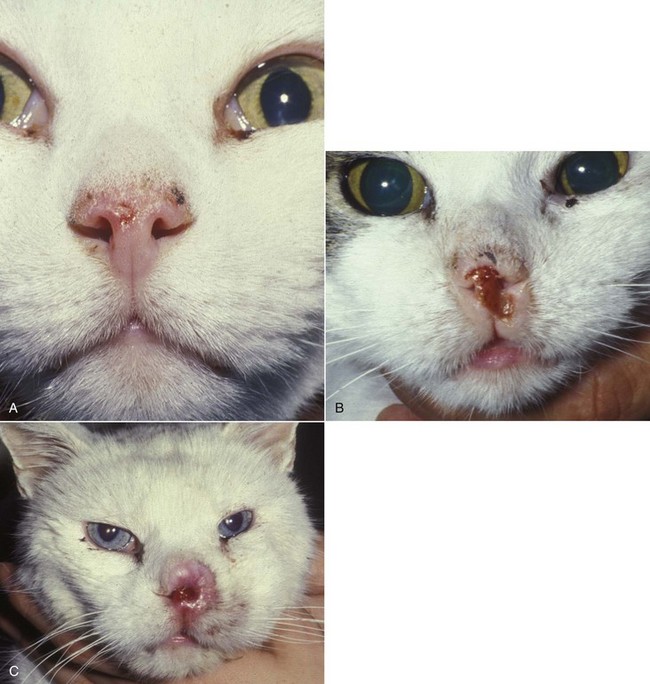
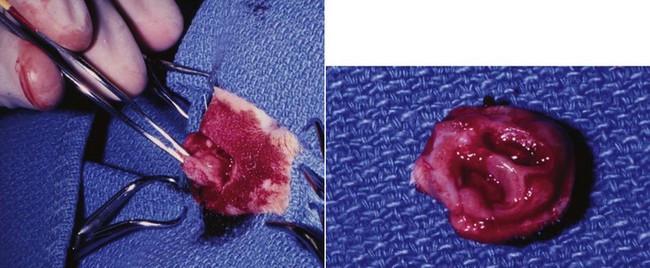
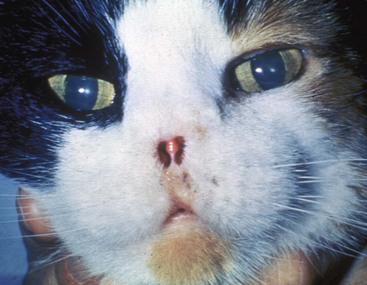
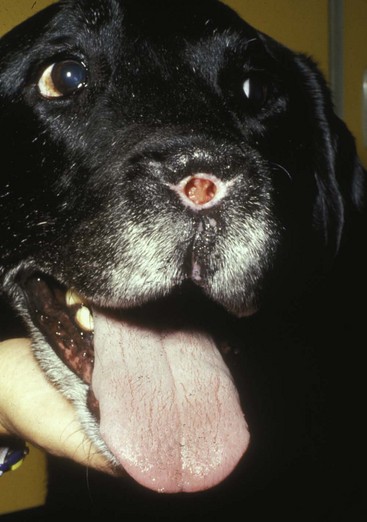
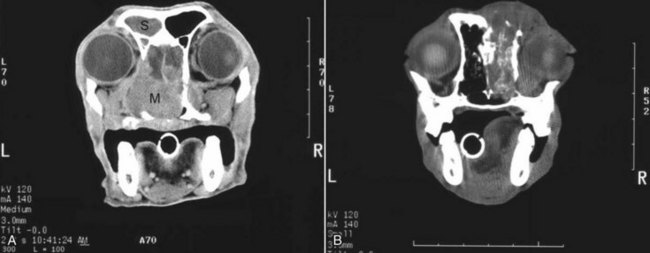
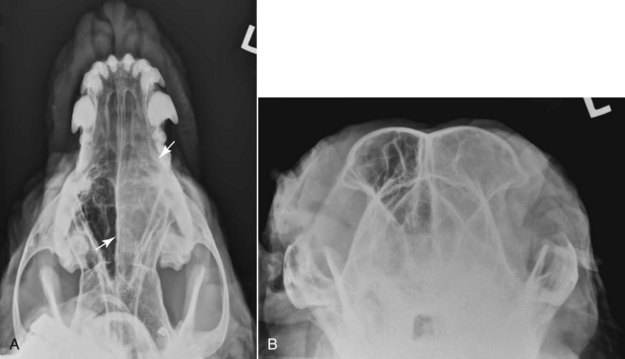
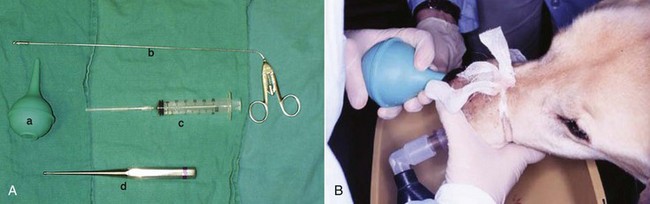
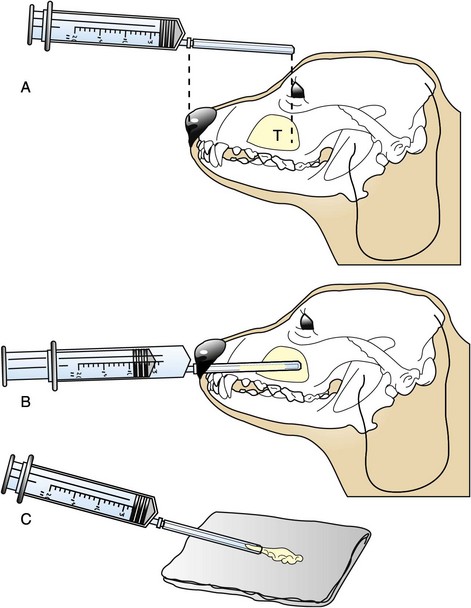
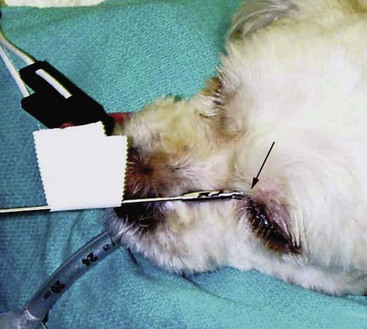
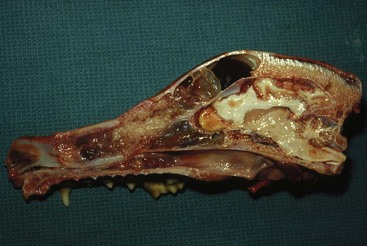
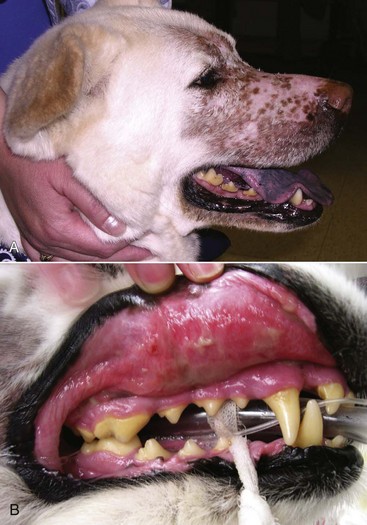
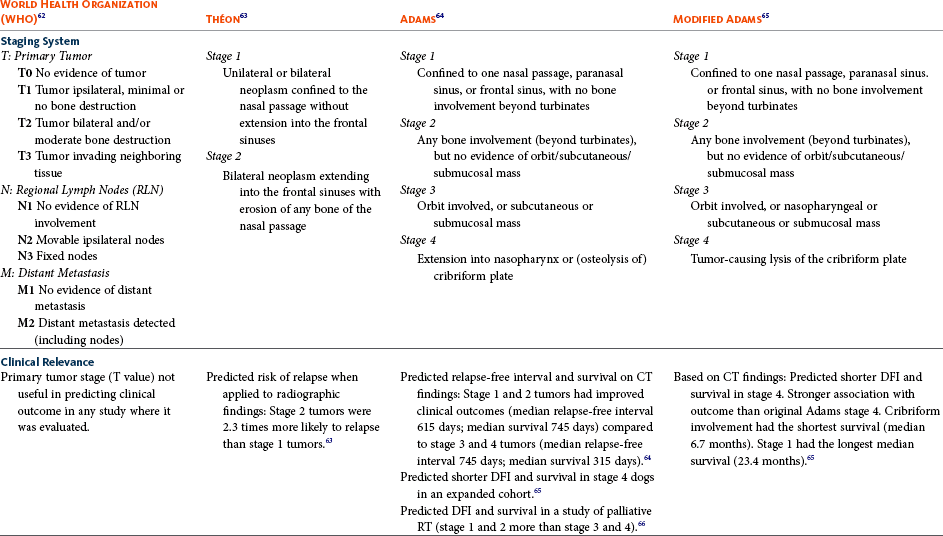
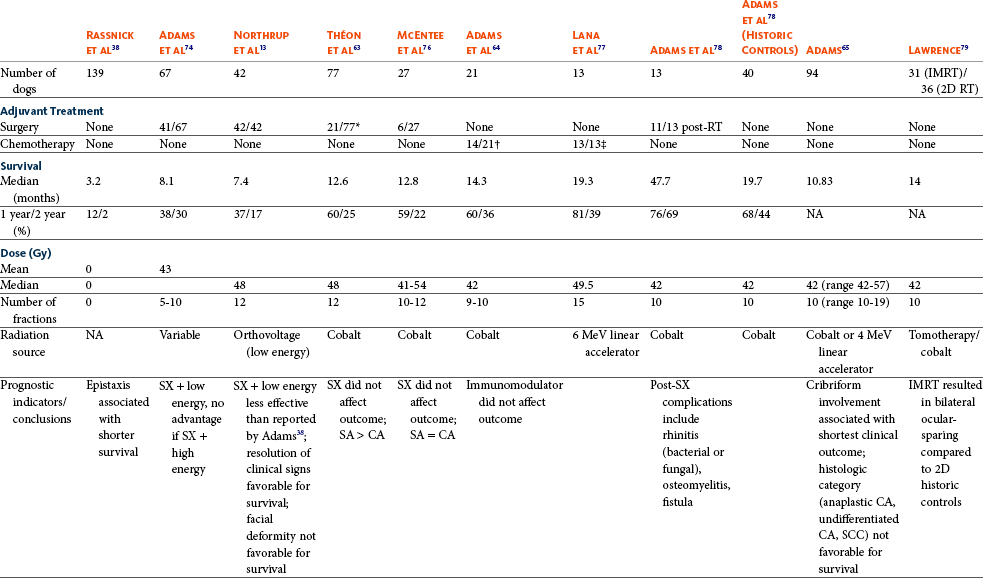
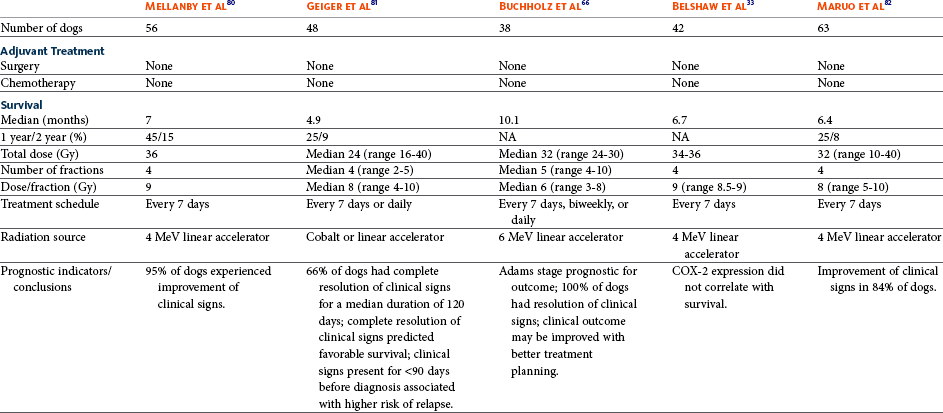
23 Cancer of the nasal planum is uncommon in the dog and relatively common in the cat. The development of squamous cell carcinoma (SCC) has been correlated with ultraviolet (UV) light exposure and lack of protective pigment.1 One paper suggests a papillomavirus may be involved in feline SCC development.2 Classically, it is seen in older, lightly pigmented cats. Invasive SCC usually is preceded by a protracted course of disease (months to years) that progresses through the following stages (Figure 23-1): crusting and erythema, superficial erosions and ulcers (carcinoma in situ or early SCC), and, finally, deeply invasive, erosive lesions. In cats SCC usually originates on the cornified external surface of the nasal planum, whereas in dogs it often occurs in the mucous membrane of the nostril or the external planum. Associated lesions on the eyelid, preauricular skin, and ear pinna may be seen in cats if these sites lack protective pigment. Patients often have been treated with corticosteroids or topical ointments, with minimal response. Limiting exposure to the sun or tattooing to add pigment protection may prevent or arrest the course of the preneoplastic disease. Topical sunscreens are easily licked off and rarely help. Maintaining the tattoo is very difficult when inflammation and ulceration are present because it is rapidly removed by macrophages. Even under the best of circumstances, tattooing must be repeated regularly and reports of success are anecdotal. Attempts to increase epithelial differentiation with synthetic derivatives of vitamin A generally have been unsuccessful for advanced disease but may be of help in reversing or limiting the growth of preneoplastic lesions.3,4 SCC and probably other neoplasms as well fall into two general categories: superficial, minimally invasive disease versus deeply infiltrating disease. Superficial cancers can be managed effectively by almost any method, including cryosurgery, lasers,5,6 photodynamic therapy,7–9 intralesional carboplatin chemotherapy,10 intralesional carboplatin combined with radiation therapy (RT),11 hyperthermia, and irradiation alone.12–19 A distinct disadvantage with these techniques is the inability to obtain a surgical margin by which to document the adequacy of treatment. RT, in particular, which would have the greatest chance of preserving the cosmetic appearance of the nose, has had poor local control rates for larger and more invasive SCC in the dog but is more effective treating SCC of the nasal planum in the cat.14,15,20 Expectations for radiation with other tumor types must be extrapolated from results achieved in more conventional sites. In the cat, invasive cancer of the nasal planum can be completely excised with an acceptable cosmetic result.21 The nasal planum is removed with a 360-degree skin incision that also transects the underlying turbinates (Figure 23-2). A single cutaneous purse-string suture of 3-0 nylon is placed to pull the skin into an open circle (1 cm in diameter) around the airways. The purse-string suture must not be pulled too tight or it may heal across the airways. The site subsequently crusts and scabs over. The scab is removed at suture removal (this often requires sedation or anesthesia), and healing of the skin with two patent airways (“nostrils”) is complete by 1 month. An occasional problem with “nosectomy” is stricture of the combined nasal orifice. This can be frustrating and has been variously managed with wide skin excision and removal of the nasal septum rostrally, laser ablation, rubber stents, or permanent placement of a stainless steel intraluminal expansile stent.22 Functional and cosmetic results are good in the cat (Figure 23-3), and fair to good in the dog23,24 (Figure 23-4). This procedure probably is the treatment of choice for invasive lesions that have not spread extensively to the lip or surrounding skin. The author has found that adjuvant RT has been successful if the margins of removal are incomplete. Combined removal of the premaxilla and nasal planum has been reported in the dog with extensive, invasive lesions.23,25,26 The outlook for SCC is good for early, noninvasive disease. However, the development of new sites of neoplasia on other areas of the planum after localized treatment is common because the underlying causes are not reversed.27 Later-stage disease can be cured with aggressive surgery but is poorly responsive to most other treatments.20,21,25 More than 80% of cats with invasive SCC of the nasal planum treated with wide resection (nosectomy) are free of recurrent disease at 1 year.5,21 Newer reports of proton irradiation16 and irradiation with intralesional chemotherapy11 suggest control rates in cats exceeding 60%. Strontium (Sr) 90 plesiotherapy can achieve 75% complete response (CR) rates for superficial SCC, and retreatment is possible. In a study of nosectomy (with or without removal of the pinnae) in 38 cats, the median survival time (MST) exceeded 22 months.5 Photodynamic therapy can be effective in treating cats with SCC of the nose, but the lesions must be small and minimally invasive for a good result.7–9 In dogs, the local recurrence rate after nosectomy is low.20,26 Delayed lymph node metastasis (longer than 1 year after surgery) in dogs with SCC has been successfully treated with lymphadenectomy. Because SCC rarely metastasizes from the nasal planum, even untreated animals with advanced cancer can live a long time, albeit with an ulcerated and deforming cancer. 1. Hargis, AM. A review of solar-induced lesions in domestic animals. Comp Cont Ed Pract Vet. 1981;3:287–294. 2. Munday, JS, Dunowska, M, De Grey, S. Detection of two different papillomaviruses within a feline cutaneous squamous cell carcinoma: case report and review of the literature. N Z Vet J. 2009;57:248–251. 3. Evans, AG, Madewell, BR, Stannard, AA. A trial of 13-cis-retinoic acid for treatment of squamous cell carcinoma and preneoplastic lesions of the head in cats. Am J Vet Res. 1985;46:2553–2557. 4. Marks, SL, Song, MD, Stannard, AA, et al. Clinical evaluation of etretinate for the treatment of canine solar-induced squamous cell carcinoma and preneoplastic lesions. J Am Acad Dermatol. 1992;27:11–16. 5. Lana, SE, Ogilvie, GK, Withrow, SJ, et al. Feline cutaneous squamous cell carcinoma of the nasal planum and the pinnae: 61 cases. J Am Anim Hosp Assoc. 1997;33:329–332. 6. Olivieri, L, Nardini, G, Pengo, G, et al. Cutaneous progressive angiomatosis on the muzzle of a dog, treated by laser photocoagulation therapy. Vet Dermatol. 2010;21:517–521. 7. Peaston, AE, Leach, MW, Higgins, RJ. Photodynamic therapy for nasal and aural squamous cell carcinoma in cats. J Am Vet Med Assoc. 1993;202:1261–1265. 8. Ferreira, I, Rahal, SC, Rocha, NS, et al. Hematoporphyrin-based photodynamic therapy for cutaneous squamous cell carcinoma in cats. Vet Dermatol. 2009;20:174–178. 9. Bexfield, NH, Stell, AJ, Gear, RN, et al. Photodynamic therapy of superficial nasal planum squamous cell carcinomas in cats: 55 cases. J Vet Intern Med/Am Coll Vet Int Med. 2008;22:1385–1389. 10. Théon, AP, Madewell, BR, Shearn, VI, et al. Prognostic factors associated with radiotherapy of squamous cell carcinoma of the nasal plane in cats. J Am Vet Med Assoc. 1995;206:991–996. 11. de Vos, JP, Burm, GO, Focker, BP. Results from the treatment of advanced stage squamous cell carcinoma of the nasal planum in cats, using a combination of intralesional carboplatin and superficial radiotherapy: a pilot study. Vet Comp Oncol. 2004;2:75–81. 12. Grier, RL, Brewer, WG, Theilen, GH. Hyperthermic treatment of superficial tumors in cats and dogs. J Am Vet Med Assoc. 1980;177:227–233. 13. Shelley, BA, Bartels, KE, Ely, RW, et al. Use of the neodymium:yttrium-aluminum-garnet laser for treatment of squamous cell carcinoma of the nasal planum in a cat. J Am Vet Med Assoc. 1992;201:756–758. 14. Carlisle, CH, Gould, S. Response of squamous cell carcinoma of the nose of the cat to treatment with x-rays. Vet Radiol. 1982;23:186–192. 15. Thrall, DE, Adams, WM. Radiotherapy of squamous cell carcinomas of the canine nasal plane. Vet Radiol. 1982;23:193–195. 16. Fidel, JL, Egger, E, Blattmann, H, et al. Proton irradiation of feline nasal planum squamous cell carcinoma using an accelerated protocol. Vet Radiol Ultrasound. 2001;42:569–575. 17. Trivillin, VA, Heber, EM, Rao, M, et al. Boron neutron capture therapy (BNCT) for the treatment of spontaneous nasal planum squamous cell carcinoma in felines. Radiat Environ Biophys. 2008;47:147–155. 18. Goodfellow, M, Hayes, A, Murphy, S, et al. A retrospective study of (90)Strontium plesiotherapy for feline squamous cell carcinoma of the nasal planum. J Feline Med Surg. 2006;8:169–176. 19. Hammond, GM, Gordon, IK, Theon, AP, et al. Evaluation of strontium Sr 90 for the treatment of superficial squamous cell carcinoma of the nasal planum in cats: 49 cases (1990-2006). J Am Vet Med Assoc. 2007;231:736–741. 20. Lascelles, BD, Parry, AT, Stidworthy, MF, et al. Squamous cell carcinoma of the nasal planum in 17 dogs. Vet Rec. 2000;147:473–476. 21. Withrow, SJ, Straw, RC. Resection of the nasal planum in nine cats and five dogs. J Am Anim Hosp Assoc. 1990;26:219–222. 22. Novo, RE, Kramek, B. Surgical repair of nasopharyngeal stenosis in a cat using a stent. J Am Anim Hosp Assoc. 1999;35:251–256. 23. Gallegos, J, Schmiedt, CW, McAnulty, JF. Cosmetic rostral nasal reconstruction after nasal planum and premaxilla resection: technique and results in two dogs. Vet Surg. 2007;36:669–674. 24. Thomson, M. Squamous cell carcinoma of the nasal planum in cats and dogs. Clin Tech Small Anim Pract. 2007;22:42–45. 25. Lascelles, BD, Henderson, RA, Sequin, B, et al. Bilateral rostral maxillectomy and nasal planectomy for large rostral maxillofacial neoplasms in six dogs and one cat. J Am Anim Hosp Assoc. 2004;40:137–146. 26. Kirpensteijn, J, Withrow, SJ, Straw, RC. Combined resection of the nasal planum and premaxilla in three dogs. Vet Surg. 1994;23:341–346. 27. Théon, AP, VanVechten, MK, Madewell, BR. Intratumoral administration of carboplatin for treatment of squamous cell carcinomas of the nasal plane in cats. Am J Vet Res. 1996;57:205–210. 28. Aasi, SZ, Leffell, DJ. Cancer of the skin. In: DeVita VT, et al, eds. Cancer: principles and practices of oncology. Philadelphia: Lippincott-Raven, 2005. Michelle M. Turek and Susan E. Lana Tumors of the nasal cavity and paranasal sinuses account for approximately 1% of all neoplasms in dogs.1 The average age of dogs with this disease is approximately 10 years, although canine patients as young as 9 months have been reported.2,3 Medium-to-large breeds may be more commonly affected.2 A slight male predilection has been suggested.2,4 It has been speculated, but is unproved, that dolichocephalic breeds (long-nosed) or dogs living in urban environments, with resultant nasal filtering of pollutants, may be at higher risk for developing nasal cancer.4–6 Exposure to environmental tobacco smoke has been associated with an increased risk of nasal cancer in a group of dogs in one study,7 but the same was not true in another.6 Indoor exposure to fossil fuel combustion products, such as those produced by coal or kerosene heaters, may contribute to the suggested environmental component of this cancer.6 Carcinomas, including adenocarcinoma, SCC, and undifferentiated carcinoma represent nearly two-thirds of canine intranasal tumors.8 Sarcomas (usually fibrosarcoma, chondrosarcoma, osteosarcoma, and undifferentiated sarcoma) comprise the bulk of the remaining cancers.9 Both carcinomas and sarcomas are characterized by progressive local invasion. The metastatic rate is generally considered low at the time of diagnosis but may be as high as 40% to 50% at the time of death, which is usually attributable to the primary disease rather than metastatic lesions.2 The most common sites of metastasis are the regional lymph nodes and the lungs.2,9,10 Less common sites include bones, kidneys, liver, skin, and brain.11–14 Rare tumors of the nasosinal region in dogs include round cell tumors, such as lymphoma (unlike in cats, in which this is a common nasal tumor); mast cell tumors; and transmissible venereal cancer. Other malignancies include hemangiosarcoma, melanoma, neuroendocrine carcinoma, nerve sheath tumor, neuroblastoma, fibrous histiocytoma, multilobular osteochondrosarcoma, hamartoma, rhabdomyosarcoma, and leiomyosarcoma.2,15–28 The biologic behavior of these less common malignancies is not well defined. Benign lesions such as polyps, fibromas, dermoid cysts, and angiomatous proliferation can also be seen. A number of studies have attempted to elucidate possible molecular mechanisms associated with canine nasosinal tumorigenesis. In one study using a single polyclonal antihuman antibody, nuclear p53 accumulation was detected in nearly 60% of nasal adenocarcinomas (11 of 19), which suggests that overexpression of a mutated p53 tumor suppressor protein may play a role.29 Cyclooxygenase-2 (COX-2) expression has been detected to varying degrees in most nasosinal epithelial tumors sampled30–33 and in normal paratumoral respiratory epithelium and stromal tissue.33 In a recent study, epidermal growth factor receptor (EGFR) expression and vascular endothelial growth factor (VEGF) expression were detected in over 50% and 90% of the 24 nasal carcinomas evaluated, respectively.34 All tumors expressed either EGFR or VEGFR, but there was no association between the immunoreactivity for each protein.34 Expression of peroxisome proliferator-activated receptor γ (PPAR-γ), a nuclear receptor involved in glucose metabolism and fatty acid storage, has also been shown in canine nasal carcinomas.35 The authors of that study suggested that expression patterns may differ in tumor tissue compared to normal nasal epithelium.35 The role of these proteins in carcinogenesis is not clear, and further investigation is needed before clinical relevancy can be determined. Evaluation of the inflammatory infiltrate in 31 canine nasal carcinomas revealed an abundance of neutrophils and macrophages, which were present in greater numbers than in normal canine mucosa.36 Plasma cells were also detected in the majority of tumors but varied in number compared to normal tissue. T-lymphocytes were detected less commonly, in lower numbers than in the normal mucosa, and predominately at the periphery of the tumors. Activity of matrix metalloproteinase-2 (MMP-2) in nasal adenocarcinomas was negligible in one small study.37 Although many intranasal diseases will have overlapping clinical signs, a strong suspicion of cancer is appropriate for older animals with an intermittent and progressive history of unilateral (initially) epistaxis or mucopurulent discharge (or both). The average duration of clinical signs prior to diagnosis is 2 to 3 months1,38; these most commonly include epistaxis, bloody or mucopurulent nasal discharge, facial deformity due to bone erosion and subcutaneous extension of tumor, unwillingness to open the mouth, sneezing, dyspnea or stertorous breathing, exophthalmus, and ocular discharge as a result of mechanical obstruction of the nasolacrimal duct.2,8,38 Differential diagnoses for animals with these clinical signs include fungal (Aspergillus sp.) or bacterial rhinitis, idiopathic nonspecific rhinitis (usually lymphoplasmacytic), rare nasal parasites, bleeding disorders, hypertension, foreign body, trauma, and developmental anomalies (e.g., cystic Rathke’s clefts).39–41 If facial deformity is present, the diagnosis is almost always cancer42,43; however, aspergillosis, sporotrichosis, and a rare, benign condition, angiomatous proliferation of the nasal cavity, also can cause facial deformity. Dogs with epistaxis and concurrent clinical signs of systemic disease, including lethargy, inappetence, weight loss, hemorrhage at extranasal sites and concurrent neurologic disease, are more likely to have nonneoplastic systemic disorders such as bleeding disorders, hypertension, and tick-borne or bacterial infections.43,44 Clinical signs can be temporarily alleviated by a variety of symptomatic treatments, including antibiotics, steroids, and nonsteroidal antiinflammatory drugs (NSAIDs).38 An initial response to these treatments should not diminish the index of suspicion for neoplasia in older dogs with clinical signs consistent with cancer.38 On rare occasions, animals with tumors involving the caudal region of the nasal cavity may have only neurologic signs (e.g., seizures, acute blindness, behavior change, paresis, circling, and obtundation) caused by direct invasion of the cranial vault.45 However, absence of neurologic signs does not rule out tumor extension into the cranial vault because most dogs with nasal tumors that extend beyond the cribriform plate do not exhibit neurologic signs. The superior imaging value of CT and MRI over conventional radiographs for canine nasal disease, including neoplasia, is well documented.3,23,39,46–52 Cross-sectional imaging provides improved anatomic detail, which allows accurate determination of the extent of tumor (staging) and localization of nasal cavity abnormalities (Figure 23-5).3,23,39,46–53 It also facilitates evaluation of the integrity of the cribriform plate and identification of potential tumor extension into the cranial vault, which are important prognostic criteria. Although, in general, MRI allows for better resolution of soft tissue structures, a recent study showed no clinically relevant benefit to using MRI over CT to evaluate nasal tumors that do not extend into the cranial vault.49 CT enables better visualization of lysis of bones bordering the nasal cavity, which is important for accurate radiation treatment planning. It has been proposed that tumor signal intensity on MRI may help distinguish sarcomas from carcinomas,50 although this application requires further validation before it can be used clinically. Both modalities are useful for imaging nasal tumors, although CT is used more commonly than MRI due to its lower cost in most facilities, wider availability, and usefulness for computer planning of radiation treatments. Certain CT or MRI findings have been correlated with a diagnosis of cancer in dogs with nasal disease.23,39,53 None of these, alone or in combination, is definitive for neoplasia.23,39,53 These findings include bony destruction (e.g., ethmoid bones, cribriform plate, and the bones surrounding the nasal cavities), destruction of the sphenoid sinus, abnormal soft tissue in the retrobulbar space, nasopharyngeal invasion, hyperostosis of the lateral maxilla, and patchy areas of increased density within abnormal soft tissue opacity.23,53 It is important to recognize that detection of a mass in the nasal cavity of a dog is not specific for a diagnosis of neoplasia.23 Idiopathic inflammatory disease and fungal infections can present in this manner.23 Conventional radiography can still have a place in the diagnostic work-up of dogs suspected of having nasal tumors. Despite the inherent limitation of tissue superimposition, the sensitivity of radiography in detecting major nasal cavity abnormalities in dogs with nasal tumors is comparable to that of CT or MRI when tumors are sufficiently advanced to cause clinical signs.46,52,54 Certain radiographic signs have been correlated with a positive predictive value for neoplasia.55 These include the presence of a soft tissue opacity, loss of turbinate detail affecting the entire ipsilateral nasal cavity, invasion of bones surrounding the nasal cavity, and soft tissue/fluid opacities within the ipsilateral frontal sinus (Figure 23-6).55 As is true for CT and MRI changes, no radiographic sign is definitively diagnostic for neoplasia.52,55 Nasal radiography therefore represents an easily accessible and less costly screening procedure that can guide further diagnostics, including biopsy, additional imaging, or both.52,56 Standard radiographs taken under anesthesia include lateral, dorsoventral (DV), frontal sinus, and open-mouth oblique views. The most informative views are the open-mouth DV oblique view (to show the caudal nasal cavity and cribriform plate) and the isolated nasal cavity exposure with the film placed in the mouth and exposed in the DV plane. A tissue biopsy should be obtained while the patient is under anesthesia for diagnostic imaging. Suitable samples can be acquired by a variety of techniques.57,58 These include vigorous nasal flushing to dislodge mass lesions, transnostril blind biopsy using cup forceps or a bone curette, fiberoptic-guided biopsy, fine-needle aspiration (FNA) or punch biopsy of facial deformities, rhinotomy, and transnostril aspiration and core biopsy (Figure 23-7). Transnostril aspiration and core biopsy involves passing either a punch biopsy needle or a large bore (3 to 5 mm) plastic cannula into the nasal cavity through the nostril and directing it to the tumor (Figure 23-8).57 With any transnostril technique, it is important to avoid penetrating the cribriform plate. The biopsy instrument should be marked with tape or cut off at a length that ensures that the instrument does not penetrate farther than the distance from the tip of the naris to the medial canthus of the eye (Figure 23-9). Mild-to-moderate hemorrhage is to be expected and subsides within several minutes. It is important to ensure the integrity and inflation of the endotracheal tube cuff during any of these procedures. If hemorrhage is severe, the ipsilateral carotid artery can be permanently ligated, but this is rarely indicated.59 Inadequate biopsy size or sampling outside the region of the tumor may preclude an accurate diagnosis. Further testing may be necessary when clinical and histologic findings are incongruent. In cases in which imaging results are suggestive of an aggressive process yet histopathologic changes are consistent with nonspecific inflammatory disease, a surgical biopsy obtained by rhinotomy may be required for definitive diagnosis.3,23 Attempts at nasal washing and fluid retrieval for cytologic examination generally have been unrewarding and are not recommended as the sole means of diagnosis.1 Brush cytology also has been described, but it often is not diagnostic.60 Rhinoscopy can be used to visualize the nasal cavity, if necessary, and to guide biopsy, although samples collected in this manner often are small and superficial.61 Multiple staging systems for canine nasosinal neoplasia have been proposed on the basis of local tumor extent and bony erosion (Table 23-1).4,63–65,67 The least clinically relevant is the World Health Organization (WHO) staging method because no correlation has been found between primary tumor extent and prognosis using this system.38,65,67,68 The prognostic significance of the other staging systems remains controversial. A recent review of 94 dogs with nasal tumors treated with definitive RT showed that the CT-based Adams staging system and its recently modified version in which stage 4 represents cribriform plate involvement alone correlate with disease outcome.65 Prognostic significance improved when CT findings were combined with histologic category.65 Table 23-1 Staging Systems for Canine Nasosinal Neoplasia CT, Computed tomography; DFI, disease-free interval; RT, radiation therapy. Regional lymph node cytology is positive for metastasis in as many as 10% to 24% of cases and is most commonly associated with carcinoma.10,38,66,68 Enlarged regional (mandibular and superficial cervical) lymph nodes should be sampled for cytology to differentiate between a reactive process and metastasis. Thoracic radiographs usually are negative for metastasis at initial presentation.1,2,10,38 Hematologic and biochemical findings generally are noncontributory in dogs with nasosinal tumors. In rare cases, paraneoplastic erythrocytosis and hypercalcemia have been documented.69–71 Therapy is directed primarily at control of local disease, which usually manifests in a relatively advanced stage in a critical location near the brain and eyes (Figure 23-10). Without treatment, the MST of dogs with nasal carcinoma is 95 days as reported in a retrospective case series of 139 dogs.38 Prognosis of dogs with epistaxis appears worse than in dogs without epistaxis (MST 88 days versus 224 days).38 Bone invasion occurs early, with nasosinal neoplasia, and curative surgery is virtually impossible. Although surgical removal by means of rhinotomy has been recommended, a high rate of morbidity without significant extension of life greatly limits the utility of this procedure as a sole form of treatment.1,8,10,72,73 The median survival time after surgery is approximately 3 to 6 months, similar to that reported for no treatment.1,10,72,73 In one study, surgery in conjunction with low-energy orthovoltage irradiation provided the best clinical outcome, MST, compared to other treatment modalities74; however, more recent reports have not supported this finding (Table 23-2).13,75 Table 23-2 *Measurable disease remained in all dogs after the surgical procedure. †Immunotherapy given using liposome-encapsulated MTP-PE. ‡Slow-release formulation of cisplatin given as chemotherapy. RT using high-energy megavoltage (MeV) equipment (cobalt source or linear accelerator) as the sole treatment modality has become the therapy of choice for canine nasosinal tumors (Table 23-3; see also Table 23-2). It has the advantage of treating the entire nasal cavity, including bone, and its use has been associated with the greatest improvement in survival. Although surgical removal of the tumor before MeV irradiation has not been shown to improve clinical outcome,10,74,83 controlled studies have not been done for this combination. MST after MeV irradiation with curative intent (i.e., full-course or definitive radiation) alone ranges from 8 to 19.7 months.* The 1- and 2-year survival rates range from 43% to 68% (1 year) and 11% to 44% (2 years).63,64,76–78,86 Doses of 42 to 54 Gy are usually delivered in 10 to 18 treatments of 3- to 4.2-Gy fractions over 2 to 4 weeks to the nasal cavity and frontal sinuses as dictated by imaging.63–65,74,77–79 True statistical comparisons between reports are not possible because of inconsistencies in methodology (even within individual reports) with respect to total dose, fraction number, dose per fraction, treatment schedules, use of CT staging, use of computerized treatment planning, response monitoring, and statistical assessment. Furthermore, differences in tumor type and tumor stage also affect patient outcome.65 CT-based computerized treatment planning can greatly enhance normal tissue sparing while ensuring optimized dose distribution within the tumor. The MST ranges from 11 to 19.7 months in dogs treated with CT-staged, computer-planned MeV irradiation to a minimum of 41 Gy.* As computer-based radiation planning becomes more advanced in veterinary medicine, it follows that clinical outcomes may continue to improve.66 Table 23-3 Summary of Selected Articles Describing Palliative Radiation Therapy for Nasosinal Tumors in Dogs RT can induce normal tissue complications in the radiation treatment field (see Chapter 12). Acute and late toxicities affect rapidly and slowly renewing tissues, respectively, and depend on daily dose to the tissue, total dose, overall treatment time, and volume of tissue treated.87 The severity of side effects can therefore vary among protocols and also between individuals on the same treatment schedule, depending on the tissues included in the radiation field. Acute toxicities associated with definitive, full-course irradiation of nasosinal tumors typically involve the oral cavity (oral mucositis), eye (keratoconjunctivitis and blepharitis), nasal cavity (rhinitis), and skin (desquamation) (Figure 23-11).63,64,76,79,88 Acute effects develop during the course of RT and resolve within 2 to 8 weeks after treatment.63,64,76,88 Oral antibiotics, pain medication, and/or ocular medication including artificial tears may be needed to support the patient during this period. Rarely, temporary esophagostomy or gastrostomy tube feedings may be indicated if oral mucositis is severe in order to maintain adequate nutritional intake. Late radiation effects, although less common than acute effects, are more detrimental and long lasting. Their development should be prevented, if possible, with thoughtful radiation treatment planning. Tissues that may be affected include the ocular lens (cataracts), cornea (keratitis, atrophy, keratoconjunctivitis sicca), anterior uvea (uveitis), retina (hemorrhage and degeneration), neuronal tissue (brain necrosis, causing neurologic changes and/or seizures, and optic nerve degeneration), bone (osteonecrosis), and skin (fibrosis).* Late complications develop months to years after therapy and are generally irreversible. The most commonly observed clinically relevant late effects in dogs treated with definitive RT for nasosinal tumors are those affecting ocular structures.68,76 Late ocular changes in the dog typically occur 6 to 9 months following RT and most often include keratoconjunctivitis sicca, cataract formation, and blindness in the irradiated eye if radiation doses are not limited.79 Other late effects are rare. Overall, although most dogs with nasosinal neoplasia experience a favorable tumor response to RT, the long-term prognosis is poor (see Table 23-2). Even when treated with a definitive (curative-intent) radiation protocol, most dogs die or are euthanized as a result of local disease progression. An investigation of treatment failure patterns following full-course MeV irradiation showed that the median duration of local control in 24 dogs was 312 days.92 Marked tumor regression (90% reduction in size) was observed using CT in 46% of cases and was associated with a longer duration of local control than that seen in dogs in which tumor response to radiation was less favorable (389 versus 161 days).92 Most of the dogs in that series experienced local progressive disease, which affirms the need for more effective treatment strategies. The following approaches have been investigated in an attempt to improve local control . 1. Full-course preoperative RT followed by surgical exenteration of residual or recurrent disease showed promise in a small series of dogs (n = 13), with a MST of 47 months compared to 19 months for dogs treated with radiation alone (see Table 23-2).78 The combination treatment was associated with an increased incidence of late effects, including rhinitis (bacterial and fungal), osteomyelitis, and fistula formation.78 A larger group of dogs must be treated in this manner to confirm these findings. 2. A logical and intuitive approach to improve local control is to increase radiation dose. This has been investigated using a boost technique in one study in which the total radiation dose was escalated to 57 Gy without an increase in overall treatment time. The treatment proved too toxic with respect to acute effects and resulted in radiation-related deaths in one-third of evaluated dogs.88 It appears that normal tissue tolerance may not allow dose escalation within standard overall treatment times using conventional radiation planning and delivery techniques. 3. The use of radiosensitizers in conjunction with ionizing radiation has been reported. A Veterinary Radiation Therapy Oncology Group (VRTOG) pilot study described the use of gemcitabine as a radiosensitizer for nasosinal carcinoma.93 Gemcitabine was given intravenously at a dosage of 50 mg/m2 twice weekly before daily RT. The authors reported significant hematologic toxicity (neutropenia) and local acute tissue complications associated with this dose and schedule. In another report, low-dose cisplatin (7.5 mg/m2 given intravenously every other day) administered in conjunction with full-course RT was well tolerated and did not appear to cause an increase in acute or late radiation effects.84 The efficacy of this approach with respect to improvement of clinical outcome is not known. A combination of radiation and cisplatin, administered intramuscularly throughout therapy using a slow-release polymer system (open cell polylactic acid polymer impregnated with platinum [OPLA-Pt]), has been shown to be well tolerated; however, survival times were similar to those in other studies that used RT alone (MST: 474 days).77 4. The goal of RT is to deliver the maximum radiation dose to the tumor while limiting the dose to surrounding normal tissues to their tolerance level.94 Currently, in veterinary medicine, maximally optimized radiation delivery is most widely achieved through the use of conventional computerized treatment planning that allows the use of multiple treatment fields directed at the patient from different angles, with differential weighting of beams, and beam shape modulators to tailor the distribution of radiation dose to each patient as much as possible. Even with the development of more advanced planning systems (i.e., 3-dimensional [3D] versus 2-dimensional [2D]), these conventional methods have limitations that prevent escalation of the radiation dose without also increasing complications.88 A potential solution to this challenge is the rapidly emerging technology called intensity-modulated RT (IMRT). IMRT achieves conformal distribution of radiation dose to the tumor while sparing sensitive normal tissues.79,95–98 Multiple radiation fields of nonuniform beam intensity are delivered with the goal of distributing the radiation dose among larger volumes of normal tissue. This results in modest (tolerable) doses to normal tissues and integration of the multiple beams into a higher total dose throughout the volume of the tumor. IMRT treatment plans are generated by “automated optimization” in which the computer itself determines the optimal nonuniform radiation exposure that must be delivered to give the desired conformal dose pattern. Through specialized software, the delivery of IMRT is achieved by using a large number of beams of varying radiation intensity that are modulated spatially and temporally.95 Modulation of beam intensity is realized using a multileaf collimator, which is a collimator that is made of multiple leaves that move rapidly and independently under computer control, customizing beam shape and intensity to the patient. As a result, IMRT has the potential to achieve a much higher degree of target conformity and normal tissue sparing than conventional (2D or 3D conformal) treatment-planning techniques.79,95–97 The complex shape of nasosinal tumors and the surrounding critical structures, including eyes and brain, provide a strong rationale for application of IMRT with this tumor type. RT-related morbidity can be significantly reduced in dogs with nasosinal tumors when IMRT is used.79,96 A recent clinical study compared radiation-induced ocular toxicity in dogs with nasal tumors treated with IMRT to that of historical controls treated with conventional 2D RT.79 IMRT reduced the radiation dose delivered to the eyes and resulted in bilateral ocular sparing. This was in contrast to profound ocular morbidity observed in the historical control group. MSTs for both groups were similar. Paramount to the success of highly conformal RT is precise daily patient positioning.95 Millimeter variation in patient setup can result in underdosing of the tumor or overdosing of normal tissues. A recent study suggested that daily setup variation associated with conventional nonrigid immobilization techniques used in veterinary RT may be insufficient to ensure high-quality IMRT delivery over a multiple-week course of treatment for nasal tumors.98 In that study, daily image guidance was necessary to ensure daily setup precision. Rigid immobilization techniques (head and cranial body fixation devices) have been developed for veterinary patients99–102 and, in conjunction with daily image guidance, permit successful implementation of highly conformal therapy.98,99 Image-guided RT (IGRT) refers to the use of “on-board” imaging, most commonly CT, integrated into IMRT delivery units. Advanced IGRT systems, such as Varian’s Trilogy, Tomotherapy, and CyberKnife, are in use at select veterinary RT facilities,98,99,103 and availability is growing. IMRT/IGRT provides the theoretical opportunity for tumor dose escalation without increasing the number of radiation treatments and overall treatment time. This in principle could translate into improved tumor control.97 A newly emerging application of IMRT/IGRT in veterinary medicine is stereotactic RT (SRT). SRT refers to the delivery of high-dose (ablative) radiation in a single dose or a few fractions.104 The treatment is delivered with extreme accuracy, and the dose is pinpointed to the tumor, minimizing the effect on nearby organs. This technique is optimally suited for small, well-defined tumor targets, although the advancement of radiation delivery technology has allowed for successful application in larger tumors.104 The high degree of precision is achieved by delivering many (up to hundreds) irregular subfields using specialized software and sophisticated multileaf collimation to create a complex intensity pattern.104 SRT fractionation schedules usually involve daily treatment for 1 to 3 days, which has obvious practical advantages compared to conventionally fractionated schedules that take weeks to complete. SRT requires highly reproducible patient immobilization, high-fidelity image guidance and an intensity modulated radiation beam to achieve extreme precision.104 Nasal tumors provide an excellent rationale for use of SRT; however, efficacy with respect to tumor control and survival rates associated with this hypofractionated treatment has not yet been proved. Initial experiences in dogs with nasal tumors include treatment with doses up to 30 Gy delivered in three fractions or in single fraction treatments of up to 15 Gy.105,106 Toxicity and tumor control data are preliminary but appear favorable.105,106 Despite the multiple apparent advantages of IMRT and SRT, the high financial costs and logistical challenges associated with optimal use of this technology must be balanced. Controlled clinical trials that rigorously examine normal tissue effects and tumor responses are needed to validate superiority of IMRT/IGRT to conventional RT in veterinary medicine. In contrast to curative-intent radiation protocols, treatment schedules that are less intensive can be used to palliate tumor-related clinical signs. The goal of palliative radiation is to improve quality of life without aiming to maximize tumor sterilization. Most palliative protocols involve coarse fractionated treatments (6 to 9 Gy) delivered weekly or biweekly.33,66,80–82 The result of this approach is temporary improvement of clinical signs in 66% to 100% of dogs and limited morbidity associated with acute side effects.66,80–82 The reported median duration of control of clinical signs ranges from 120 to 300 days.66,81 Acute toxicities affecting the oral mucosa and skin are generally mild and short lived.66,80–82 A multiinstitutional retrospective study reported development of chronic ocular toxicities in 13% of 39 dogs that had at least one eye irradiated.81 Reported MSTs range from 146 days to 300 days.33,66,80–82 Tumor stage (Adams stage I) and duration of clinical signs (>90 days) have been correlated with longer survival in cases receiving this type of radiation.66,81 While true statistical comparisons between studies are not possible because of inconsistencies in methodology, the best clinical outcome in terms of duration of resolution of clinical signs and survival (approximately 300 days for both) is reported in a study of 38 dogs in which radiation treatment planning was performed using CT-staging and 3D-conformal computerized treatment planning software.66 In contrast to other studies, radiation delivery was planned using standardized dose and volume specifications ensuring uniform tumor dose distributions. The authors suggested that sophisticated radiation treatment planning in canine nasal tumor patients treated palliatively may result in more favorable clinical outcomes than previously reported.66 A recent report suggested that some dogs with recurrent nasal tumors can experience a second clinical remission following reirradiation of the tumor.107 The study evaluated a selected cohort of nine dogs in which the median time to tumor progression after a first full course of RT was 513 days. The first course of radiation delivered a median of 50 Gy in a median of 18 fractions. The median total dose used in the second protocol was 36 Gy, delivered in approximately 2-Gy fractions. The overall MST for this small, selection-biased cohort of dogs was 927 days (95% confidence interval [CI] 423 days to 1767 days). All dogs developed one or more late effects involving the skin, eyes, and nasal cavity. Late effects in seven of the nine dogs were considered mild and did not affect quality of life. Severe late effects were reported in two dogs, and both had sudden blindness that led to euthanasia. The authors concluded that there is a population of canine nasal tumor patients (i.e., those with slowly progressive tumors) that will benefit from reirradiation. Further work is needed to refine time-dose prescriptions to minimize radiation-induced late effects. Conformal techniques such as IMRT could play a role in this setting to limit morbidity. Chemotherapy is used rarely as a sole therapy for canine nasosinal tumors. Treatment with cisplatin alone has been shown to benefit some dogs.108 A clinical response rate of 27%, including one radiographically confirmed complete remission, was reported in a small series of 11 dogs.108 All of the dogs experienced alleviation of clinical signs, and the MST was 5 months. Another report evaluated a combination chemotherapy protocol of doxorubicin, carboplatin, and oral piroxicam in eight dogs with advanced nasal tumors.109 A clinical response rate of 75% was observed, including 4 CRs confirmed by CT imaging. All dogs experienced resolution of clinical signs, and the protocol was well tolerated. MST in these eight dogs was 210 days (range 150 days to 960 days). These preliminary results are favorable, but the case number is small and more dogs need to be treated in this manner to confirm these findings. Other therapeutic approaches to nasal tumors that have been investigated include proton-beam therapy, brachytherapy, immunotherapy, cryotherapy, and photodynamic therapy (PDT). Charged particles like protons have a well-defined tissue range and sharp dose fall off, which could potentially be exploited for conformal tumor targeting and normal tissue sparing.110 A small clinical trial involving nine dogs with nasal tumors treated with proton-beam therapy resulted in tumor responses and survival times similar to those reported for MeV irradiation.110 Acute effects of the skin and eyes were pronounced in some dogs, and 50% of dogs developed radiation-induced cataracts. Due to limited availability of proton irradiators, this technology is unlikely to be optimized for use in dogs. Intracavitary radiation using radioactive isotopes (brachytherapy) has been evaluated after surgical removal of nasosinal tumors in dogs.111,112 Potential problems associated with this type of radiation include dose distribution and radiation exposure to personnel. The question of whether brachytherapy improves survival over traditional external-beam radiation has not been answered. Immunotherapy and cryosurgery have not improved survival times.1,113 A recent case report described the use of image-guided cryotherapy in the treatment of a rapidly recurrent nasal carcinoma that resulted in long-term tumor control and survival.114 The authors suggested that further investigation of this technique may be warranted for the management of focal residual or recurrent nasal tumors. Reports evaluating PDT have been published; however, results are too preliminary to draw any conclusions.115,116 When all treatments fail to control epistaxis, unilateral or bilateral carotid artery ligation can palliate the symptoms in dogs for up to 3 months or longer without damage to the brain.58 The importance of prognostic factors in the treatment of canine nasosinal tumors remains controversial. Negative predictors of survival from various studies include age (>10 years),68 epistaxis,38 duration of clinical signs,81 advanced local tumor stage,63–66,68 metastatic disease,10,68 histologic subtype (carcinoma, particularly squamous cell or undifferentiated),63,65,74 and failure to achieve resolution of clinical signs.81 An analysis of 94 dogs with varying subtypes of nasal carcinoma or sarcoma treated with curative RT at three veterinary facilities was recently performed.65 A correlation was demonstrated between clinical outcome and the original Adams tumor staging scheme,64 as well as the modified Adams tumor system.65 Based on these findings, dogs with cribriform plate involvement as determined by CT imaging have the shortest disease-free survival (DFS) and overall survival (OS). This subset of dogs had a median DFS of 3.8 months and a median OS of almost 7 months, compared to dogs with unilateral tumors without bone involvement in which DFS and OS were 6.5 and 23 months, respectively. The study also showed that prognosis varied based on histologic category. When grouped together, dogs with anaplastic carcinoma, undifferentiated carcinoma, and SCC had a shorter DFS. There was no effect on clinical outcome when all carcinomas were compared with all sarcomas. The statistically strongest association with clinical outcome was made when clinical stage was combined with histologic findings. A major pitfall of many veterinary studies with respect to assessment of treatment efficacy in canine nasosinal cancer is the evaluable endpoint. Tumor response and time to tumor progression are the most representative measures of treatment efficacy. Regular diagnostic imaging, ideally CT or MRI, would be required to allow accurate determination of these endpoints. Due to high costs associated with imaging and the need for anesthesia, follow-up is rarely done in this manner. Analyzing the return of clinical signs as an indication of tumor recurrence is problematic since similar signs can result from rhinitis secondary to therapy (radiation and/or surgery) or residual tumor.117 Assessment of the survival time, as is commonly done in most veterinary nasosinal cancer studies, may be biased by the use of additional treatments on suspicion of progressive disease and by the decision for euthanasia, which can vary greatly from one pet owner to another. Furthermore, inconsistencies in methodology between studies and even within individual reports, as well as lack of controlled studies, are other limitations that have prevented the informed development of the optimal treatment approach to nasosinal tumors in dogs.
Tumors of the Respiratory System
 Section A
Section A
Cancer of the Nasal Planum
Incidence and Risk Factors
History and Clinical Signs
Therapy
Prognosis
References
 Section B
Section B
Nasosinal Tumors
Canine Nasosinal Tumors
Pathology and Natural Behavior
History and Clinical Signs
Diagnosis and Staging
Treatment and Prognosis
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Tumors of the Respiratory System