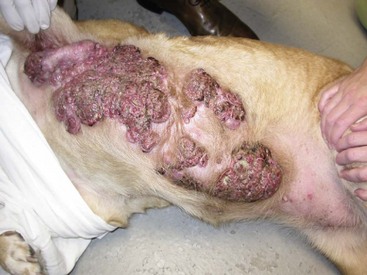
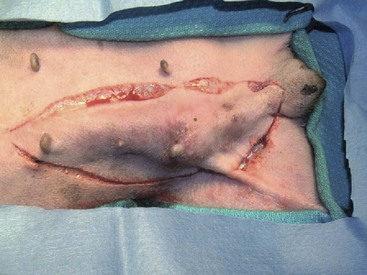
27 Mammary gland tumors are common in dogs and represent the most common neoplasms in sexually intact female dogs.1–6 The incidence rates reported vary, depending on the origin of the studies and characteristics of the source population. The current incidence of mammary tumors in the United States is lower than in many other countries due to the common practice of performing ovariohysterectomy (OHE) at a young age. Data from several European national or regional canine cancer registries, including Norway, Denmark, and Italy, provide information regarding tumor incidence in general, as well as details regarding the relative frequency of various tumors according to site, age, and breed. These registries consist of a population of predominantly sexually intact dogs and thus provide insight into the natural or true mammary tumor risk in unaltered dogs. Results from these registries show that mammary tumors are the most common tumors in female dogs and represent 50% to 70% of all tumors in this subset of the population.2,6 In general, open population-based and insurance-based studies may underestimate the true incidence of disease, especially if the diagnosis and subsequent registration require a surgical biopsy. Furthermore, the insured dog population may be skewed toward younger animals because of age restriction and may be void after the tenth year of age, which coincides with the peak incidence age of mammary tumor diagnosis.3,4 Early data from the surveys of Alameda and Contra Costa counties reported an estimated annual incidence rate of 257.7 malignant mammary tumors per 100,000 in intact female dogs.1 A more recent large Swedish study based on 80,000 insured female dogs, most of which were sexually intact, reported a rate of 111 mammary tumors (including both benign and malignant) per 10,000 dog-years at risk.3 This study also reported an increasing risk for tumors with advancing age; 6% of all 8-year-old dogs and 13% of all 10-year-old dogs were diagnosed with at least one mammary tumor. Another large insurance-based study from the United Kingdom reported an annual incidence rate of 205 mammary tumors per 100,000 dogs. This study included all mammary tumors regardless of histology.4 In addition to these open and more heterogeneous population-based studies, closed population studies provide another source of incidence and natural progression data. Longitudinal studies may provide a more accurate estimate of the total lifetime risk of mammary tumors because dogs are monitored closely and all tumors are noted, biopsied, and reported. In a large beagle colony morbidity and mortality study, 71% of female dogs developed at least one mammary gland neoplasm in their lifetime.7 However, this may not accurately represent the incidence in other breeds. Many of the various tumor registries have reported significant breed variations in mammary tumor incidence, suggesting that, in addition to age and hormonal factors, hereditary breed-associated genetic susceptibility also contributes to mammary tumor risk. Mammary tumors affect middle-aged and older dogs.1,8–11 Mammary tumors, especially malignant tumors, are extremely rare in dogs younger than 5 years old.1,8,12 The tumor risk increases with age and becomes significant when dogs turn 7 or 8 years old and continues to increase until the age of 11 to 13 years.8,12 Dogs with malignant tumors have been found to be significantly older than dogs with benign tumors: mean age of dogs with malignant tumors is 9 to 11 years and 7 to 9 years with benign tumors.13,14 The peak incidence age also depends on the lifespan of various breeds. In general, larger breeds have a naturally shorter lifespan and therefore tend to be younger than smaller breeds when they are diagnosed. These differences may be further exaggerated in high-risk breeds such as the English Springer spaniels.3,15 Many mammary tumors in dogs are preventable. Dogs spayed prior to their first estrus have only a 0.5 % risk of developing mammary tumors in their lifetime.16 The protective effect of OHE decreases quickly over the first few estrus cycles, and most studies have not found significant benefit after 4 years of age. According to Schneider’s original study, the risk increases and the benefit diminishes with each estrus cycle, as illustrated by an increasing risk of 8% and 26%, depending on whether the OHE was performed prior to the second or third heat cycle.16 This study found no significant risk reduction in dogs spayed after the second heat cycle, although other researchers have found some modest benefit in dogs spayed later.12,17,18 There is general agreement that the greatest benefit on mammary tumor prevention is seen if the dog is not allowed to go through any heat cycles, suggesting that the pivotal and irreversible effects of ovarian hormones on the mammary glands in terms of cancer risk occur early in life, likely during puberty when the mammary gland develops and matures. These findings may also explain why other factors resulting in physiologic variation in hormonal influence on the mammary tissues such as pseudopregnancy, pregnancy, or parity, which typically occur after a few estrus cycles, have not been found to significantly influence the tumor risk.12,16,19 Exposure to exogenous or pharmacologic doses of hormones (both progestins and estrogens), however, has been found to increase the risk for developing mammary tumors in dogs. Dogs treated with progestins are more likely to develop tumors and are younger when they do. According to the Norwegian Canine Cancer Registry, dogs treated with progestins to prevent estrus had a 2.3 times higher risk for mammary tumors when compared to dogs not receiving such treatment.20 Similarly, a Dutch study found that privately owned dogs with mammary tumors were significantly more likely to have received progestins.18 Numerous studies have investigated the effect of dose, duration, and type of hormones (progestins, estrogens, or combination of both) on mammary tumor development in laboratory dogs. Although some discordance exists, most conclude that low-dose progestins alone increase the risk for predominantly benign tumors, whereas a combination of estrogens and progestins tends to induce malignant tumors.21–25 In general, mammary tumors tend to be more common in the smaller breeds. Purebred dogs are more commonly affected1; poodles, Chihuahuas, dachshunds, Yorkshire terriers, Maltese, and cocker spaniels are frequently listed as high-risk breeds in the small-breed category.1,2,14,26 However, some of the larger breeds are also at increased risk, including the English Springer spaniel, English setters, Brittany spaniels, German shepherds, Pointers, Dobermans, and Boxers.1–3,14,26 Some noteworthy discrepancies exist, specifically between the U.S. and the European reports. Boxers are noted to have a decreased risk for mammary tumors according to data from the University of Pennsylvania, whereas Scandinavian studies reflect an increased risk in Boxers.2,3,14 A closed population beagle study also has shown that two different lines or families of beagles have very different mammary tumor risk.27 These results collectively support a genetic influence on mammary cancer development. Familial or inherited germline mutations in BRCA1 and BRCA2 account for 5% to 10 % of all human breast cancers and are associated with an 85% cumulative lifetime risk of breast cancer in affected individuals.28–31 Studies of BRCA mutations in canine mammary tumors have so far been limited to tumor gene expression studies and the results have varied; some found underexpression of BRCA1 in malignant tumors and others have documented overexpression of BRCA2 in metastatic tumors.32,33 Germline mutations in both BRCA1 and BRCA2 were found to be associated with significantly increased risk in English Springer spaniels in a large Swedish study.3,15 Body weight, specifically during puberty (9 to 12 months), is found to have a significant effect on later mammary tumor risk; being underweight during this time period provides significant protection against later tumor development.17 This study did not find an increased risk for tumors in dogs fed a high-fat diet or dogs that were obese around the time of tumor detection. However, a subsequent case-control study did document an association between diet and mammary cancer in which obesity early in life and a diet high in red meat were found to increase risk.34 Obesity has also been recognized as a risk factor for developing postmenopausal breast cancer in women.35,36 One of the proposed mechanisms by which diet/obesity may be linked to breast carcinogenesis is via its effect on serum estrogen levels. Obesity is associated with decreased concentration of sex hormone–binding globulin and thus results in elevated serum free estrogen levels.37–41 In addition, adipose tissues may be a source of increased estrogen production via aromatase-mediated conversion of androgens. Interestingly, the mammary cancer–sparing influence of being underweight is most significant during the first year of life when the effects of the endogenous hormones are the greatest. Based on the previous discussion of risk factors, it is clear that exposure to ovarian hormones is important in the development of mammary tumors in dogs. Both estrogens and progesterone are necessary for normal mammary gland development and maturation. The mammary glands undergo distinct clinical and histopathologic changes as hormone levels fluctuate according to the phases of the estrus cycle.42,43 Estrogens and progesterone are mitogens of breast epithelium and induce proliferation of intralobular ductal epithelium and development of ducts and lobules, resulting in expansion of the mammary glands. Historically, the tumorigenic effects of estrogen in human breast cancer were thought to be mediated via their receptor binding and enhanced production of growth factors resulting in increased cellular proliferation.44 However, more recent research shows that estrogen and its metabolites also have direct genotoxic effects by increasing mutations and induction of aneuploidy independent of the estrogen receptors.45–47 The tumorigenic effects of progesterone are in part thought to be mediated via a progesterone-induced increased mammary gland production of growth hormone (GH) and growth hormone receptors (GHR).48–50 GH has direct stimulatory effects on mammary tissues, as well as indirect effects via increasing insulin-like growth factor-1 (IGF-1).51 The GH/IGF-1 axis has been implicated in human breast carcinogenesis. IGF-1 is both a proliferative and a survival factor for breast epithelial cells and regulates the expression of numerous genes involved in breast cancer development.52–57 The complex dysregulation of growth factors and hormones that precedes, initiates, and potentially drives canine mammary tumorigenesis is far from understood; evidence exists indicating that both growth factors and steroid hormones are intrinsically implicated and contribute in an autocrine/paracrine manner. Malignant tumors have significantly higher tissue concentrations of GH, IGF-1, progesterone, and 17β-estradiol than benign tumors, and moreover, levels correspond with important clinicopathologic parameters, such as growth rate, size, and specific histopathologic type.58,59 A complete review of the biologic and molecular aspects of mammary tumor carcinogenesis is beyond the scope of this chapter, but recent reviews provide more complete information.60,61 The entire mammary chains are exposed to growth factors and sex hormones, resulting in a field carcinogenesis effect. Consequently, most dogs develop tumors in multiple glands.7,12,13,62–64 Histologic progression with increasing tumor size is often noted in dogs with multiple tumors and areas of transitions such as carcinoma in situ can be seen in benign tumors.13,62 This provides direct evidence that benign and malignant mammary tumors are not separate entities—instead, they are part of a biologic and histopathologic continuum in which the malignant invasive carcinomas are the endstage of the process. Earlier publications support this hypothesis and document associations between tumors of benign and malignant histology. For instance, dogs with carcinomas often had concurrent benign tumors of the same cell type and dogs with benign mammary tumors were at increased risk for developing subsequent malignant tumors.63 Furthermore, risk was even higher in dogs diagnosed with carcinomas or carcinoma in situ.13,62,64 Evidence of histologic progression has also been reported in which a high incidence of carcinoma in situ and intraepithelial lesions with atypia was noted adjacent to invasive carcinomas.62,65 These studies provide support for the hypothesis that mammary tumors develop initially from benign lesions and progress to invasive malignant lesions as part of a continuum influenced by hormonal field effects on mammary tissues. There are likely regional variations in terms of exposure, resulting in a range of histopathologic and clinical changes. Some tumors may never change and remain small and benign, whereas others progress and become malignant and many develop new tumors in other glands. This suggests that canine mammary tumors provide unique comparative opportunities to study mammary carcinogenesis and progression with direct applications to human breast cancer research. Hormonal exposure plays an important role in mammary tumor development, and many tumors, specifically tumors of epithelial origin, express hormone receptors (HRs), suggesting continued hormonal influence and dependence. Benign tumors are more likely than malignant tumors to retain HRs—both estrogen receptors (ER) and progesterone receptors (PR).66–70 The HR status is also influenced by age and hormonal status: dogs that are intact, younger, and in estrus are more likely to have receptor-positive tumors than dogs that are spayed, older, and anestrous.70,71 Furthermore, the HR expression is inversely correlated with tumor size and histopathologic differentiation; larger tumors and undifferentiated or anaplastic tumors are less likely to express receptors than tumors with more differentiated histology, reflecting a biologic drift toward hormone independence and corresponding with aggressive histology and clinical behavior.71–73 HR expression analysis is most commonly performed by immunohistochemistry (IHC). Results from various studies are quite disparate, especially in terms of ER-alpha positivity in malignant tumors, and range from 10% to 92%.66–74 These variations may in part be due to differences between study populations (tumor size, castration status, tumor types) and the fact that IHC methods vary and are neither standardized nor validated. This makes it difficult to consider HR expression results when making treatment decisions. Despite these discrepancies, several studies have documented that tumor expression of ER and PR is associated with a more favorable outcome.71–73 Endocrine therapy is recommended to all women with ER-positive tumors, regardless of intensity of staining, and results in significant improvement in the adjuvant setting.75–80 Currently, there are no published prospective studies on the predictive value of HR status and the effect or benefit of hormonal therapy in dogs with mammary tumors. Until results from such studies are available and found to be predictive, routine IHC for HRs is not likely to influence treatment decisions. In addition to the presence of HRs, the overexpression of human epidermal growth factor receptor-2 (HER2/erb-2), a member of the epidermal growth factor receptor (EGFR) family involved in signal transduction pathways that regulate cell growth and differentiation, may also provide clinical and prognostic insight, as well as therapeutic opportunities in mammary tumors. Overexpression or amplification of HER2 is found in 20% to 25% of all human breast cancer patients and is associated with aggressive behavior, resistance to hormonal therapy, and a poor prognosis.81–83 HER2 overexpression has also been documented in canine mammary tumors using the same HercepTest scoring systems used in human breast cancer and documented positive staining ranging from 17% to 29% in malignant tumors.84,85 HER2 staining was associated with negative histologic features and short survival according to one of these studies,84 but contrary to the human studies, HER2 expression was associated with an increased survival in two other independent studies.85,86 Mammary tumors are usually easy to detect through routine physical examinations. However, high-risk dogs, specifically older intact female dogs, should undergo a thorough examination of the mammary glands. Mammary tumors typically affect the two caudal pairs, where the mammary glands or tissues are naturally larger; thus careful palpation may be necessary to detect small tumors.7,12,63,64 The mammary glands should be palpated again under general anesthesia to ensure that all tumors are found and included in the surgical planning, and both chains should be carefully evaluated. A recent study documented that 70% of intact females had more than one tumor at diagnosis.13 The size of the tumor(s), stage of disease, and presence of systemic signs of illness vary widely. Inflammatory mammary carcinomas represent a rare but clinically important subset of mammary tumors in dogs. Affected dogs may easily be misdiagnosed as having mastitis or severe dermatitis because, rather than presenting with discrete well-circumscribed tumors, the entire mammary chain may appear edematous, swollen, warm, and painful (Figure 27-1).87,88 In addition to the extensive locoregional involvement, most dogs with inflammatory carcinomas have distant metastatic disease and signs of systemic illness.87,88 These dogs are therefore poor surgical candidates. The majority of dogs with mammary tumors are systemically healthy, and the tumors are confined to the mammary glands when they are diagnosed. Due to the risk of metastasis associated with mammary tumors, staging prior to initiating therapy is strongly recommended, especially if benign disease cannot be histologically confirmed. Minimal staging can include complete blood count (CBC), serum biochemistry, three-view thoracic radiographs, and fine-needle aspiration (FNA) of regional lymph nodes, even if palpably normal. Abdominal ultrasound may be indicated in dogs with suspected regional lymph node involvement or changes on preoperative blood work suggesting tumor-related or non–tumor-related serum biochemistry changes. Even though osseous metaplasia occurs occasionally with mammary adenocarcinoma and the mammary glands are a common site for extraskeletal osteosarcoma, there has been no prognostic value found for serum alkaline phosphatase activity.89,90 There may be value in performing mammary tumor cytology to help rule out nonmammary dermal and subcutaneous tumors (e.g., mast cell tumors, lipomas). Additionally, correlations between cytopathology and ex vivo histopathology have been reported to be between 67.5% and 93%,91,92 and the reported cytologic sensitivity and specificity for a malignant mammary tumor diagnosis were 88% and 96%, respectively.93 Computed tomography (CT) imaging of the thoracic cavity provides more sensitive detection of pulmonary nodules than does thoracic radiography, but this may not be applicable for every patient due to a need for general anesthesia, the increased expense, and decreased availability.93,94 Distant metastatic sites can include lymph nodes, liver, lungs, and bone.95 Lymphatic drainage of normal mammary glands is very complex, with documented drainage occurring to multiple ipsilateral lymph nodes and even to contralateral lymph nodes.96–98 The amount of variation in lymphatic drainage increases in the neoplastic mammary gland.96 Tumor-induced lymphangiogenesis, well documented in human breast cancer, may be responsible for the unpredictable and erratic location of susceptible or “at-risk” lymph nodes in dogs having malignant mammary tumors.99 Thus exclusive anatomic sampling of nearby lymph nodes is not sufficient for accurate lymph node staging and may miss the presence of locoregional disease.100 Mammary tumors are staged according to the T (tumor), N (lymph node), and M (metastasis) system. A modified version of the original staging system published by Owens101 is currently used by most oncologists and stage advances from I to II to III as the size of the primary tumor increases from smaller than 3 cm, to between 3 and 5 cm, to larger than 5 cm.102 These size categories capture important changes in prognosis and outcome. Lymph node metastasis represents stage IV disease, regardless of tumor size, and distant metastasis constitutes stage V disease. This staging system should be used for dogs with epithelial tumors (noninflammatory) and not sarcomas (Table 27-1). Table 27-1 Staging of Canine Mammary Tumors Data from Rutteman G, Withrow S, MacEwen E: Tumors of the mammary gland. In Withrow S, MacEwen E, editors: Small animal clinical oncology, ed 3, Philadelphia, 2001, WB Saunders. The normal mammary gland is a complex branching structure and the histologic and immunohistochemical characteristics are equally complex—the interested reader is referred to a thorough review on the subject.103 Two early classifications of canine and feline mammary tumors were published in 1974104 and 1999,105 and a revised system for the dog was published in 2011.106 In dogs, although there may be histopathologic evidence of malignancy, only a small percentage of cases will have lymphatic and vascular invasion and metastatic disease, whereas in cats the majority are malignant and metastasis to local lymph nodes is more common. The classification used in this text is based on both morphology106 and prognosis.107 When discussing the classification of mammary neoplasms the terms simple and complex are commonly used. Simple denotes that the neoplasm is composed of one cell type resembling either luminal epithelial cells or myoepithelial cells, whereas complex neoplasms are composed of two cell types, both luminal and myoepithelial cells.106 Several hyperplastic and dysplastic lesions are considered precursor lesions to the development of mammary neoplasms.106 These include duct ectasis, lobular hyperplasia (with or without secretory activity), lobular hyperplasia with fibrosis, epitheliosis, papillomatosis, fibroses (fibrosclerosis), and fibroadenomatous change (fibroepithelial hyperplasia, fibroepithelial hypertrophy, mammary hypertrophy). Several types of benign mammary neoplasms exist in the dog and include adenoma, intraductal papillary adenoma (duct papilloma), ductal adenoma, fibroadenoma, myoepithelioma, complex adenoma (adenomyoepithelioma), and benign mixed tumors. A histologic description of these various entities is beyond the scope of this chapter, and interested readers are directed to a more thorough review.106 Several types of malignant epithelial tumors (Table 27-2) exist, including carcinoma in situ (well-demarcated, noninfiltrative nodule[s] that have not extended through the basement membrane) and a variety of carcinomas. In addition, several less common subtypes of malignant epithelial neoplasms are squamous cell carcinomas, adenosquamous carcinomas, mucinous carcinomas, lipid-rich carcinomas, and spindle cell carcinomas (including malignant myoepithelioma, squamous cell carcinoma–spindle cell variant, and carcinoma–spindle cell variant).106 Malignant mesenchymal neoplasms include osteosarcoma, chondrosarcoma, fibrosarcoma, hemangiosarcoma, and carcinosarcoma (malignant mixed mammary tumor).106 The epithelial tumors are also graded according to specific histopathologic criteria. Several systems for both canine and feline tumors exist, most of which are based on the Elston and Ellis grading system,108 which incorporates information regarding (1) tubule formation, (2) nuclear pleomorphism, and (3) mitosis per 10 HPF (Table 27-3).109–111 Based on the total score derived from this system, the grade of the tumor will be determined: grade 1 (low score) is a well-differentiated tumor, grade 2 (intermediate score) is moderately differentiated, and grade 3 (high total score) is poorly differentiated (Table 27-4). Tumor grade has been found to provide consistent and reliable prognostic information.71,106–111 In addition to the grading system, information regarding vascular/lymphatic invasion, surrounding stromal invasion, lymph node involvement, and tumor type may also predict behavior.* Sarcomas are typically not graded according to this system, but the majority tend to be biologically aggressive tumors and associated with a very poor long-term survival.111,112 Table 27-3 Criteria Used for Histologic Grading of Malignancy in Feline and Canine Mammary Carcinomas Table 27-4 Tumor Grade Based on Histologic Score in Felines and Canines Data from Misdorp W: Tumors of the mammary gland. In Meuten DJ, editor: Tumors in domestic animals, ed 4, Ames, Iowa, 2002, Iowa State Press; Clemente M, Perez-Alenza MD, Illera JC, et al: Histological, immunohistological, and ultrastructural description of vasculogenic mimicry in canine mammary cancer, Vet Pathol 47:265-274, 2010; and Castagnaro M, Casalone C, Bozzetta E, et al: Tumour grading and the one-year post-surgical prognosis in feline mammary carcinomas, J Comp Pathol 119:263-275, 1998. Mammary tumors in dogs represent a wide histologic spectrum with both benign and malignant lesions originating from different tissue types or a combination of tissues. Many dogs present with several different tumors and tumors of different types and can as such represent a rather daunting histopathologic picture; prognosis is determined by the most aggressive tumor, and decisions regarding adjuvant treatments should be based on the largest or the most aggressive histology. In many cases, the most aggressive tumor is the largest.13 According to MacEwen et al, dogs with tumor volume larger than 40 cc (approximately 3.4 cm in diameter) have a statistically significant worse outcome than smaller tumors, both in terms of remission and survival.114 Other investigators have classified tumors as stage I, smaller than 3 cm; stage II, between 3 cm and 5 cm; and stage III, larger than 5 cm.101,114 Dogs with tumors smaller than 3 cm were reported to have a significantly longer survival.11,71,115 Others, however, have found that a change in prognosis only becomes significant when tumors are larger than 5 cm.26,116 The change in prognosis is likely gradual as tumors increase in size. The modified WHO staging system has incorporated these three size categories representing stage I, stage II, and stage III, respectively.102 These stages are commonly used in mammary tumor staging. Importantly, however, the size of the tumor becomes irrelevant if the local lymph node is involved; according to Kurzman et al, the size of the primary tumor was not significant in dogs with local lymph node involvement.11 A positive lymph node constitutes stage IV disease according to the revised WHO system, attributing a worse prognosis to lymph node involvement rather than tumor size. A large retrospective study, including only dogs with carcinomas, all of which had the local or draining lymph node removed and biopsied, found that the status of the local lymph nodes was highly prognostic.11 Others have confirmed these findings.* Therefore information regarding the status of the local lymph node is extremely important when considering the need for adjuvant or systemic therapy in dogs with mammary tumors. Both the original and the revised WHO staging system provide prognostic information. When performing a side-by-side comparison of the two systems, the revised system appears to better reflect the stronger impact of lymph node status on prognosis.103 Nevertheless, the original staging system also provides useful prognostic information as illustrated in two larger separate retrospective studies in which dogs with higher WHO stage disease had a significantly worse prognosis than dogs with lower stage disease.26,117 Several studies have evaluated the effect of surgical dose in canine mammary tumors. A prospective randomized trial of 144 dogs with naïve malignant tumors comparing the overall survival benefit and disease-free interval (DFI) relative to either chain mastectomy or simple mastectomy found no differences.114 Similarly, a retrospective case series of 79 dogs treated at a single institution found no difference in overall survival or DFI comparable to the type of surgical procedure performed, whether lumpectomy, mastectomy, regional mastectomy with en bloc lymph node excision, or chain mastectomy with en bloc lymph node excision.117 However, the relative hazard for death within the first 2 years after surgery was slightly higher for dogs receiving a regional mastectomy over a chain mastectomy.117 Interestingly, in the study by MacEwen et al, the hazard curves for DFI and survival were quite similar, suggesting that most dogs that experienced recurrence developed metastasis and not new tumors. However, the rate of new tumors was not reported in this study.114 A differing study indicates that surgical “dose” is important. In this case series of 99 dogs, all intact female dogs underwent either a regional or chain mastectomy for a single mammary gland tumor with unknown histology.118 Of these, 58% of dogs developed a new tumor in the remaining ipsilateral mammary gland tissue following a regional mastectomy and those whose initial tumor was subsequently determined to be malignant were more likely to develop an ipsilateral tumor. The authors advocated for an initial unilateral chain mastectomy for female intact dogs with a single mammary tumor, although, in their population, 42% of dogs did not develop a subsequent tumor and would have experienced a larger surgical dose than needed.118 Unfortunately, other large useful studies investigating the association between OHE and survival did not report on the completeness or extent of mammary tumor removal.18,119,120 Development of second mammary tumors is well documented and has been reported in over 70% of dogs with malignant mammary tumors following lumpectomy, although the impact of second mammary tumor development on survival is not clear.16,114,118 It seems intuitive that a single standardized guideline for surgical treatment omits consideration of factors such as the dog’s age, tumor size, tumor number, previous mammary tumors, and stage and may not provide the optimal outcome. Future carefully constructed clinical trials may offer more tailored recommendations based on the individual patient’s risk. Current recommendations based on available data suggest that for dogs with a single mammary tumor of known or unknown histotype, surgical excision wide enough to completely remove the mammary gland tumor is adequate. Incomplete excision or cytoreductive procedures are not endorsed.95 Tumors that are fixed or have skin ulceration and are less than 1 cm in diameter may be sufficiently managed with a mammectomy (Figure 27-2).95 “Wide excision” has not been well defined, but for larger tumors, this may be generalized to a 2-cm lateral margin and modified according to the size of the patient and tumor.95 The deep margin may need to include the abdominal muscular fascia and/or portions of the abdominal wall to be excised en bloc with the mammary tumor, depending on size and fixation.95 If abdominal surgery is to be performed simultaneously for OHE, penetration of the tumor prior to abdominal entry is to be avoided to prevent direct spread of tumor cells; rather, tumor removal should follow abdominal closure. For animals with multiple mammary tumors, more extensive resections such as a regional mastectomy or unilateral chain mastectomy may need to be pursued. As with other tumor resections, surgical margin assessment is critical for malignant mammary tumors, and additional surgery should be pursued if incompletely excised. Elective unilateral or bilateral chain mastectomies may be reasonable for young intact bitches with multiple tumors because there is a suggestion for development of additional tumors (Figure 27-3).95 In spite of the evidence for recurrent mammary gland tumor development, there is no sufficiently compelling evidence at this time for routine recommendation of complete, unilateral or bilateral chain mastectomies.
Tumors of the Mammary Gland
Mammary Gland Tumors in Dogs
Epidemiology
Age
Hormonal Exposure
Breeds and Genetic Susceptibility
Other Risk Factors
Tumor Biology: Development, Hormones, Growth Factors, and Clinical Implications
Tumor Hormone Receptors: Prognostic, Clinical, and Therapeutic Implications
History and Clinical Presentation
Clinical Assessment, Diagnosis, Work-Up, and Staging
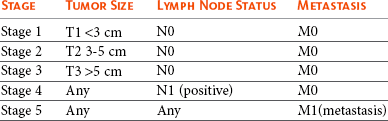
Staging System
Histopathology
Classification Systems
Canine Mammary Hyperplasia and Dysplasia
Benign Mammary Neoplasms
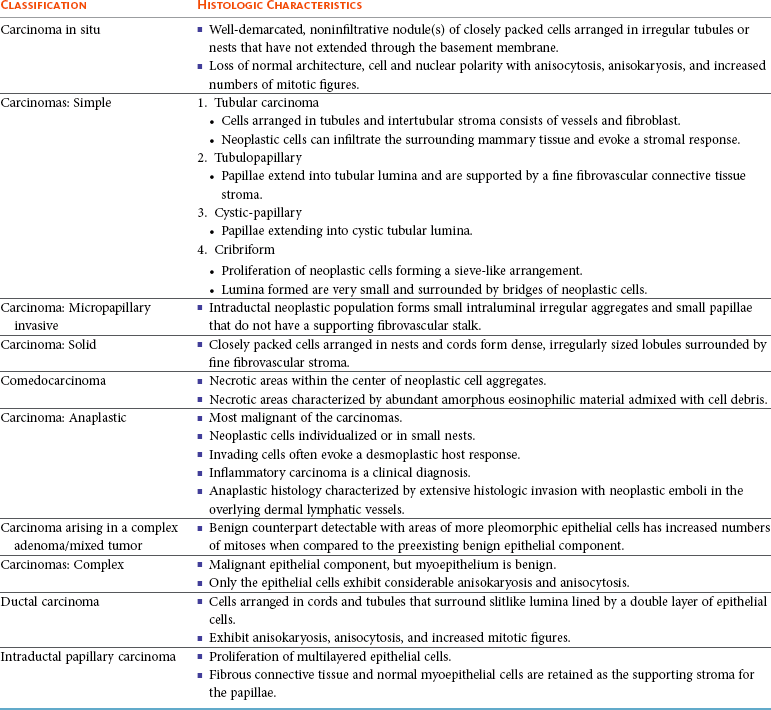
Malignant Canine Mammary Neoplasms
Malignant Mesenchymal Neoplasms: Sarcomas
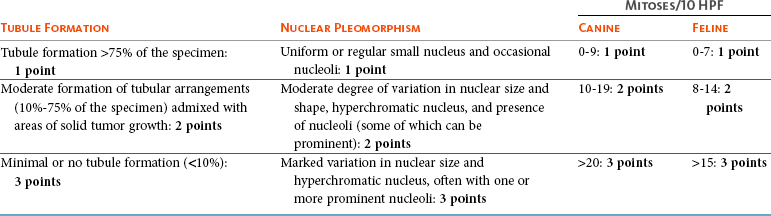
Histopathologic Prognostic Factors and Grading
Total Score
Grade of Malignancy
3 to 5
I (low)
Well differentiated
6 to 7
II (intermediate)
Moderately differentiated
8 to 9
III (high)
Poorly differentiated
Clinical Prognostic Factors
Tumor Size
Lymph Node
WHO Staging System
Therapy
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Tumors of the Mammary Gland