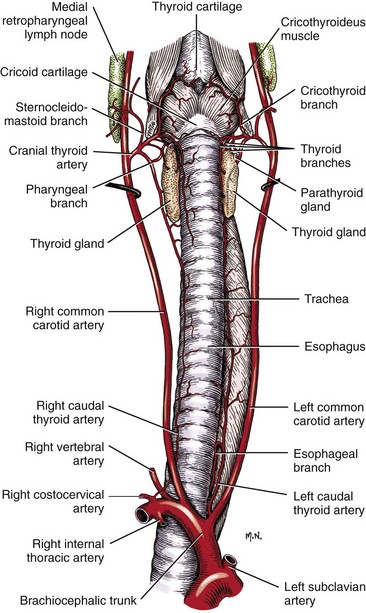
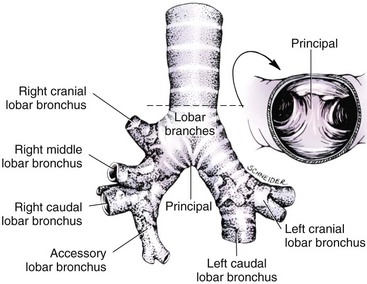
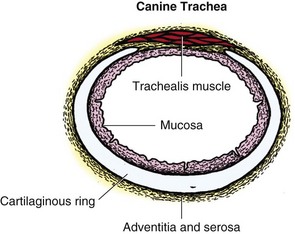
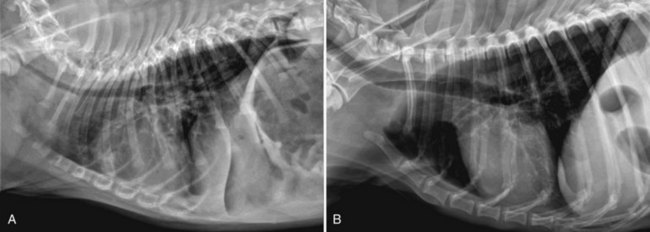
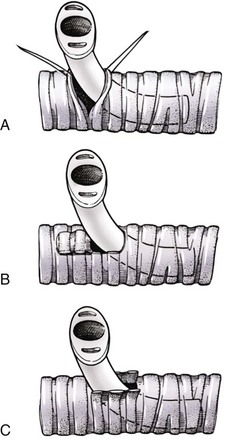
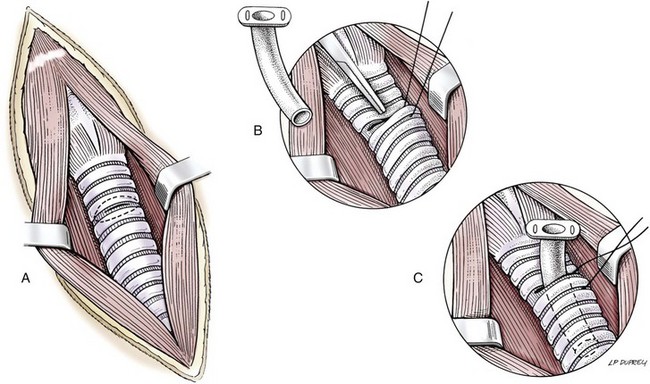
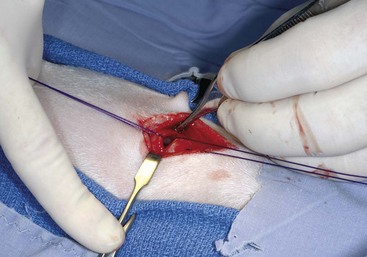
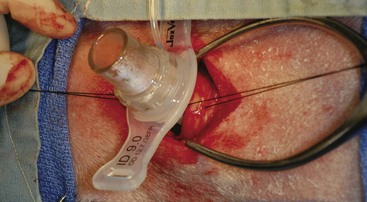
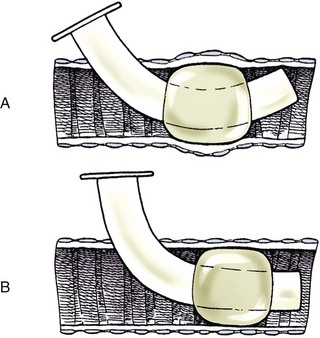
Chapter 102 The cricotracheal junction arbitrarily marks the border of the upper and lower respiratory tracts. The trachea extends from the cricoid cartilage of the larynx to the base of the heart, where it terminates at the carina (Figure 102-1). At this point, the trachea bifurcates into the principal (mainstem) bronchi, one to each hemithorax.1,63,65 These then split into lobar bronchi, one of which supplies each anatomic lung lobe (Figure 102-2). Segmental bronchi branch from these and further divide into subsegmental bronchi and eventually bronchioles.5,35,63,65 It has been estimated that dogs have between 17,000 and 36,000 terminal bronchioles.99 The trachea consists of incomplete hyaline cartilage rings, which are connected dorsally by the trachealis muscle (Figure 102-3). The cartilage arches serve to exert outward pressure against the trachealis muscle, thereby maintaining the tracheal lumen.177 Approximately 35 rings are present, and a range of up to 46 has been reported.36,50,53,59 Between the rings of the trachea are bands of fibroelastic tissue known as the annular ligaments, which are continuous with the perichondrium.63 These supporting soft tissue structures allow remarkable shape and length accommodation of the more rigid tracheal rings. The left mainstem bronchus is typically composed of three rings; the right mainstem bronchus has only one.67 The smaller bronchi contain overlapping cartilage plates, rather than rings, and the disappearance of these plates marks the transition into bronchioles.63 The tracheal wall in cross-section consists of an inner mucosa and submucosa followed by a fibrocartilaginous ring and outer adventitia in the cervical trachea or serosa in the intrathoracic trachea.63 The lumen of the canine trachea is roughly circular, with a normal tracheal width to height ratio of 1 : 1.53 Each tracheal ring is thickest ventrally and tapers toward the tip.50 The trachealis muscle is composed primarily of transversely oriented smooth muscle fibers and connects to the two arms of the tracheal rings on their external surfaces in both dogs and cats.39,40,63 Histologically, cartilage of the tracheal rings is thinnest at the thoracic inlet, and the lumen in this region has the least cross-sectional area.50 Computed tomography (CT) has also confirmed that the tracheal lumen is widest at the level of the cricothyroid junction and progressively tapers to its narrowest point at the thoracic inlet into the thoracic trachea.107,108 The trachea and principal bronchi are lined by pseudostratified columnar epithelium resting on a basal lamina.8,23,111,172 Ciliated columnar cells, goblet cells, basal cells, and smaller numbers of endocrine cells are present within this layer.111 Beneath the epithelium is the lamina propria–tunica submucosa, which contains tubuloalveolar mucus glands.8,111 These glands are present throughout the trachea in dogs, averaging one glandular opening per millimeter2 ventrally and decreasing dorsally.40 Tubuloalveolar glands are highly productive, with a single structure supplying as much mucus as 40 goblet cells.172 During progression down the bronchial tree, the tubuloalveolar mucus glands disappear first, the goblet cells become much less frequent, and finally ciliated cells disappear.8 The trachea receives a segmental blood supply from the cranial and caudal thyroid arteries. Vascular structures can be seen coursing through the soft tissue pedicles to each side of the trachea, and arteriolar branches are grossly visible between the tracheal rings. These branches travel toward the midline, where they extensively anastomose.118 Extending from these arterioles is a rich subepithelial mucosal plexus. In deeper mucosa, this is a plexus of venules and arterioles. Closer to the mucosal basement membrane is an extensive capillary network.118 At the level of the carina, the source of blood supply shifts primarily to the bronchoesophageal arteries. Venous drainage is via the thyroid, internal jugular, and bronchoesophageal veins.45 Lymph flows to the deep cervical, cranial mediastinal, medial retropharyngeal, and tracheobronchial nodes.45,172 The main innervation of the tracheal mucosa and trachealis muscle is from the right vagus and recurrent laryngeal nerve.46,151 The trachea serves multiple functions aside from serving as a conduit of inspired gases to the lungs. Although not a primary function, the trachea assists the upper airway in conditioning of inspired air. During forced inspiration of cool air, circulation to the respiratory mucosa is increased, which provides additional warmth and humidification.7,57,159 In a similar vein, the bronchial circulation has an important role in maintenance of mucosal hydration.159 More vital to airway homeostasis is the function of the mucociliary escalator. Inspired particulate matter becomes entrapped within mucous secretions in the tracheobronchial tree and is transported to the larynx via coordinated ciliary action.26 The cilia generally beat in a direction parallel to the length of the trachea and become oblique around obstructions such as the vocal folds and bronchial openings.92 The mucous blanket accumulates in the aryepiglottic regions of the larynx until it is expelled and swallowed. A typical mucociliary flow rate of 10 to 15 mm per minute in dogs was discovered using inhaled radioactive microspheres and tantalum dust.26 The rate of escalation differs among species and has been reported to average between 20 to 30 mm/min in animals ranging from a hen to an ox when evaluated in vitro using fine particulate matter.94 The speed and efficiency of ciliary transport are hindered with increasing particle size and with escalating mucus viscosity.94 General anesthetics can also slow or stop the ciliary beat.26 Smooth muscle control to the trachea is provided by the vagus nerves. Maximal stimulation of the left vagus nerve results in only 28.5% of the contraction achieved by maximal stimulation of the right. Therefore, the right vagal efferents are presumed to be dominant in dogs.46 Active contraction of tracheobronchial muscle causes the airways to become less distensible and less compressible.144,145,148 As the arms of the cartilage rings are drawn together during contraction, the membranous trachea folds in a furrow. This infolding makes the trachea less distensible because the portion of tracheal lumen bound by membranous tissue is decreased by one half to two thirds.43,114 Similarly, the greater proportion of lumen surrounded by the tightening cartilage rings increases rigidity of the structure, making it less compressible. Incremental increases in external pressure collapse the trachea when the trachealis muscle is flaccid but maintain luminal patency during contraction.144,145 This phenomenon is structurally related to protection of the membranous portion of the trachea by the overlapping cartilage arms.145 A direct result of smooth muscle contraction is increased airflow velocity within the trachea. As rigidity increases proportionally with the degree of trachealis contraction, precise control of airflow can be achieved.148 This increase in velocity is even more pronounced in a compressed trachea, as would be present intrathoracically during expiration.44 As pressure outside of the airway increases, a degree of dynamic compression ensues. Interestingly, flow through the trachea exhibits hysteresis, depending on this pressure.115 When the trachealis muscle is contracted, however, this effect may be reduced because of infolding of membranous tissue.43,114 The invagination of the membranous portion of the trachea that ensues from active contraction of the trachealis muscle forms a furrow along which mucus can be collected and expelled. Along with the fact that airflow is proportionally increased, secretions are mobilized and moved. This has been described as a “squeegee” effect and is likely used during expulsion of viscous secretions with the cough reflex.42 High-quality thoracic radiographs can be used to identify lesions, evaluate the thorax for other abnormalities, and survey whether additional studies are needed.158 The trachea is easily visualized on thoracic films because of the sharp interface of soft tissue and air. The trachea is located just to the right of the midline in the cranial mediastinum. On lateral radiographs, it should travel roughly parallel to the spine, diverging slightly in the thorax where the vertebrae angle dorsally and deviating ventrally at the carina.113 In normal animals, the lumen of the trachea should remain uniform in all phases of respiration. It is important to distinguish the phase of respiration when attempting to use thoracic radiography to demonstrate dynamic processes.34,158 To minimize artifact from positioning, the head should be placed in a neutral position.113 The tracheal size can be evaluated by comparing the tracheal diameter with the thoracic inlet diameter.84,113 In nonbrachycephalic breeds, the mean ratio is 0.20 ± 0.03. In brachycephalic breeds, the mean ratio is slightly less at 0.16 ± 0.03, with the exception of English Bulldogs, which have a mean ratio of 0.13 ± 0.38 (Figure 102-4). Fluoroscopy can be particularly helpful when evaluating dynamic changes in tracheal diameter because it provides a real-time record of function.34,158 As the patient is examined in an awake state, permutations such as coughing can be elicited and observed. Fluoroscopy can also be useful for interventional techniques, such as tracheal foreign body retrieval and tracheal stent placement.168,176,184 CT allows for the construction of three-dimensional radiographic images. The most obvious downfall of CT is the need for general anesthesia,158 often limiting its use in respiratory distress cases. CT evaluation of normal dogs has been reported, and the utility of this technique for other areas is currently being explored.107,108,120 In clinical cases, CT is often used for radiation planning and has been used to diagnose the site of a tracheal rupture.22 Endoscopic anatomy of the bronchi has been reported for dogs and cats.5,35 Tracheobronchoscopy is a versatile tool because it can be used for diagnosis of functional lesions, biopsy of mechanical lesions, and removal of foreign bodies and to document disease progression or response to treatment.34,87 It is the most reliable method for diagnosing and grading airway collapse and evaluating the bronchial tree and is required for the diagnosis of bronchomalacia.105 Bronchoalveolar lavage through a bronchoscope is the only means by which directed samples can be retrieved from the airway for cytology, culture, and sensitivity. Transtracheal lavage or endotracheal lavage can be used to obtain generalized, nonguided samples. Temporary tube tracheostomy is indicated for various conditions that result in upper airway obstruction, such as trauma, anaphylaxis, neoplasia, and conformational defects. In addition, this technique has proved useful for planned airway management during surgical manipulation of the oral cavity.* Although temporary tracheostomy may be performed under absolute emergency conditions, it is preferable to stabilize the patient, secure the airway with an endotracheal tube, and provide ventilatory support under general anesthesia. In the most critical patients, a method of percutaneous tracheostomy placement (Seldinger technique) has been used in dogs.45,161 A myriad of surgical techniques for tube placement have been described (Figure 102-5). None has been shown to be particularly advantageous with respect to functionality or the incidence of complications.31,45 The tracheal incision should be as limited as possible while allowing insertion of an appropriate tube and should therefore approximate the tube diameter.10 The tube size should be large enough to provide adequate airflow through the tube but small enough to allow ample room for airflow around the tube. This helps to prevent silent death from occlusion of the tube lumen.45,84,88 After a tracheostomy has been performed, intensive management and 24-hour observation of the patient are essential. A ready assortment of supplies for tube management should be maintained near the patient at all times.71 If possible, a nurse should be designated to the patient to ensure that subtle changes in condition are noted because deterioration can occur rapidly.45,84,88 A ventral cervical midline approach is made through the skin and subcutaneous tissues (Figure 102-6). The sternohyoideus muscles are separated at their midline aponeurosis to expose the ventral trachea. An incision is created either between two tracheal rings in a horizontal (transverse) fashion or through two to three rings in a vertical (longitudinal) fashion. The length of the transverse incision should not exceed half the circumference of the trachea. Depending on the orientation, stay sutures are placed around the tracheal rings adjacent to the incision (horizontal) or through adjacent rings (vertical) to assist in maintenance of luminal patency (Figure 102-7). These sutures are clearly labeled and will later facilitate mobilization of the site for tube exchange. An appropriate tube is then gently inserted into the tracheal lumen (Figure 102-8). The tube is secured around the neck using umbilical tape. The stoma around the tube is coated in a thin layer of antibiotic ointment, and the neck is lightly bandaged to protect the stoma. The cuff of the tracheostomy tube should not be inflated unless positive-pressure ventilation is required or anesthetic gases are being administered.45,88,122 When inflation is necessary, the minimal occluding volume should be used to decrease the incidence of mucosal damage; that is, the least volume to hold a positive ventilatory pressure of 15 cm H2O (Figure 102-9).45 The cuff should be deflated and the tube repositioned every 4 hours to allow mucosal reperfusion and relieve potential stress on the tracheal wall. Because it bypasses the upper airway, tracheostomy results in inspiration of cool, dry air. The tracheobronchial tree responds by increasing mucus production, which collects around and within the tube and stoma. Whereas single-lumen tubes must be removed and replaced every time cleaning is necessary, double-lumen tubes have a removable cannula that can simply be exchanged. At a minimum, tubes should be replaced twice daily; exchange can be required as frequently as every 4 to 6 hours after initial placement because of excess airway secretions.45,79,166 Tubes should be cleaned and soaked in 2% chlorhexidine and rinsed with sterile saline before replacement. Airway suctioning is another means used to keep the tube patent and is performed as frequently as necessary after tube introduction. A period of preoxygenation before suctioning is recommended, as is the use of a sterile suction catheter. The suction should be applied for no more than 10 to 12 seconds at a time and then released.45,71 Uninterrupted suction can lead to severe atelectasis and hypoxia. A vagal response may also be elicited, resulting in gagging, retching, or bradycardia.71,88 Therefore, electrocardiographic monitoring during the suction procedure is recommended. Immediately after completion, the animal should again receive supplemental oxygen. To ensure adequate humidification of the airway, 0.2 mL/kg of sterile saline is administered through the tube every 1 to 4 hours or nebulization is used.45 Coupage and multiple controlled periods of exercise on a harness and leash can be used to mobilize secretions. Parenteral antibiotics are typically not continued beyond the operative period if there is not a preexisting lower airway malady. The bandage must be changed daily and the peristomal region monitored for infection.45,71,88 In veterinary medicine, most cannulations are of short duration (hours to days).84 The decision to remove the tube typically coincides with resolution of the inciting event. Before tube removal, it is prudent to occlude the tube (assuming air can pass around it) and stoma with an occlusive dressing for 15 to 20 minutes under direct observation to ensure that respiratory distress does not ensue.45 Tracheostomy wounds heal by second intention within 7 to 10 days.83,164 During this time, owners are instructed to clean the stoma of secretions at least twice daily and apply antibiotic ointment to prevent concretions of exudate around the stoma. During the healing process, a degree of stenosis occurs at all temporary tracheostomy sites.101 Immediate complications occur in approximately 50% of patients and include plugging of the tube with debris, inadvertent tube removal, gagging, coughing, vomiting, subcutaneous emphysema, pneumomediastinum, pneumothorax, infection, and respiratory distress.79,84,166 The most common complication is plugging of the tube (Figure 102-10), which occurs in 18% of cases.84 It has been theorized that cats may form more mucus and have a higher chance of tube occlusion. In a study of cats with temporary tracheostomy tubes, 87% had complications, 40% of which were life threatening. A total of 91% of cats with benign disease were discharged from the hospital with no further complications.79 Although respiratory secretions are the most common cause of airway occlusion, a recurrent tracheal thrombus has also been described secondary to temporary tracheostomy tube placement in a dog.72 Figure 102-10 View of an occluded tracheostomy tube. (Photograph courtesy Delbert J. Krahwinkel, Jr., DVM, MS, DACVS, DACVA, DACVECC.) The most significant long-term complication of tracheostomy is stenosis. In humans, 11.0% to 17.8% of patients experience significant stomal stenosis.6,10 The mere placement of a tube increases the incidence of stomal stenosis compared with operations without tube placement.10 In addition, larger tubes in humans have been correlated with the degree of stenosis.6 Increased pressure against tracheal rings causes cartilage erosion and results in a generous stoma, regardless of the initial incision size.31 This is presumably a result of cartilage deformation and the action of inflammatory cells at the site from pressure against the rings.6,122 Two areas of stenosis have been identified—at the stoma site itself and at the level of the cuff or tube tip.122 In a prospective study of humans, functionally significant complications occurred in 17.8% of subjects; however, all patients with adequate radiographs exhibited some degree of subclinical stenosis.6 This is likely also true in dogs, in which an average loss of 25% of luminal area at 60 days was reported, regardless of the type of tracheostomy created.31 The type of cuff used can also contribute to complications. Tracheal damage at the cuff site occurs relatively early in the course of intubation and includes mucosal erosions that extend to the cartilage within the first 48 hours.39 Healing of these defects by a cicatricial scar invariably causes stenosis. The advent of high-volume, low-pressure cuffs has reduced the incidence of tracheal damage.39 In a low-volume, high-pressure cuff, 1 mL of air was shown to exert 55 mm Hg of outward pressure on the trachea6 compared with 2.5 mm Hg of outward pressure with 2 mL of air in a high-volume, low-pressure system.39 Therefore, when cuffs are necessary, use of low-pressure cuffs is preferred. In humans, pressure from tracheostomy tube cuffs has been so severe that tracheoesophageal fistulae and carotid artery erosion have been reported.6,39 Permanent (tubeless) tracheostomies are defined as those in which a stable stoma is created by suturing tracheal mucosa to the skin.89 The most common indications for permanent tracheostomy are laryngeal masses in cats and laryngeal paralysis or collapse in dogs.79,90,166 Other indications include irresolvable trauma and laryngeal resection or reconstruction. A ventral midline skin incision is created, and the sternohyoideus muscles are separated on the midline (Figure 102-11). The sternohyoideus muscles can be apposed dorsally to the trachea using mattress sutures to help elevate the trachea closer to skin level or retained lateral to the trachea with suture.88 The ventral half of the cartilage of three or four tracheal rings is removed with a #11 blade and the mucosa incised. Perimucosal tracheal tissue can be attached to the dermis to help relieve residual tension before mucocutaneous apposition. A simple interrupted or continuous pattern of fine (3-0 or 4-0) absorbable suture material is used to appose the mucosa to the skin. Suture bites are placed approximately 2 mm apart to create the tracheostoma (Figure 102-12).88–90 Immediate complications are similar to those of tube tracheostomy. In a retrospective study of 21 cats with permanent tracheostomies, mucus plugs within the respiratory tree were the most common complication, leading to death by asphyxiation in five cats. The overall median survival times in two reports were 20.5 days166 and 42 days.79 Stenosis of the stoma is a long-term concern and can be minimized with careful attention to technique because healing via granulation causes more stenosis than primary mucosa to skin healing.90 Gross anatomic analysis of 16 permanent tracheostomy sites in dogs 9 to 23 months after creation revealed stenosis, which was less than 10%, in only three animals.12 Hedlund et al.89 reported stenosis of up to 60% of diameter, with all animals affected to some degree, and recommended planning preoperative stoma size with stricture in mind. A degree of caution should be exercised because tracheal collapse may occur if the stoma is too large.45,88 Similarly, if there is preexisting collapse, the stoma site should be reinforced with extraluminal polypropylene rings. Healing of mucosa to skin occurs over 2 weeks.88 During this period, cleaning of secretions should be gentle to minimize disruption of the surgical site. Typically, after 1 month, secretions are minimal.90 Care of the tracheostoma is similar to that of temporary tube tracheostomies (described previously). Initially, the stoma needs cleaning and suctioning every 4 to 6 hours45,166 and should be protected from secretions with a thin layer of antibiotic ointment. Because skin fold occlusion is a prime concern, excision of skin folds may be helpful in maintaining patency.90 The tracheal mucosa undergoes squamous metaplasia and contains inflammatory infiltrates at 0 to 4 weeks after permanent tracheostomy, becoming more normal by 16 weeks.89 It is important to recognize that a permanent orifice and bypass of the upper respiratory system have been created; therefore, environmental modifications are necessary for a successful outcome. Airway irritants should be minimized to lessen secretions, and animals should be kept at a healthy weight to ease exercise and shrink excess folds. Animals should be walked with harnesses rather than collars or any restraint on the neck. It is important to keep the hair clipped around the stoma, and any opportunity for swimming should be avoided because this may result in drowning.45,88 Small incisions or wounds to the trachea or bronchi are typically closed in a simple interrupted fashion using a mattress pattern or other tension-relieving suture.147 Mucosal wounds begin to epithelialize within 2 to 8 hours. By 48 hours, a transitional epithelium arises.172 Transformation into ciliated and goblet cells can begin at about 96 hours.172 Granulation tissue is minimized by accurate alignment, use of minimally reactive suture, and gentle tissue handling.172 Large defects of tracheobronchial mucosa may not heal as quickly, however, and any healing through granulation tissue may lead to stricture.67 Anesthesia: Anesthetic administration, specifically the adequate delivery of oxygen, must be planned for each stage of the procedure. Improper planning may lead to periods of hypoxia, which may become life threatening. Sterile endotracheal tubes and anesthetic circuits should be available to surgeons for intraoperative transincisional intubation as needed.87 Tension: The primary principle of anastomosis is to provide precise anatomic apposition under minimal tension to encourage rapid healing with a low chance of infection.56,70 Although as many as 15 to 27 tracheal rings have been resected in dogs under experimental conditions,16,52 tension is considered to be the limiting factor for long tracheal resections. Increasing tension serves to displace the anastomotic site, resulting in healing via granulation tissue formation rather than primary epithelialization.87 Although the respiratory mucosa begins to heal small defects rapidly, larger areas can take substantially longer. A 1- × 1-cm defect takes 15 to 20 days to epithelialize in dogs.67 Various reports have demonstrated an increased incidence of severe stenosis with increasing tension. To produce clinical signs, 50% to 75% luminal stenosis is required.15 At a tension of more than 750 g, stenosis invariably results.119,178 At double this tension, circumferential strictures were documented. There is inherent primary tension on the trachea because of its suspension in elastic peritracheal tissues, resulting in displacement of its ends when the trachea is cut.16 Because of this phenomenon and the subsequent risk for stenosis, various tension-relieving mechanisms have been suggested, including tension-relieving sutures placed several rings proximal and distal to the anastomosis, fixed ventroflexion of the neck, and release of the annular ligaments with concurrent preservation of mucosa.52,56,88,119,178 Tension-relieving sutures have been shown to negate tension at anastomotic sites subjected to forces greater than 2000 g.178 In a recent study examining anastomoses under static distraction, both simple continuous and interrupted sutures reinforced with a tension-relieving horizontal mattress pattern distributed tension better and had a higher resistance to suture pullout than a simple interrupted pattern incorporating the tracheal rings.56 Much of the current literature regarding experimental work on tracheal resection has been performed in sheep because of their availability, ease of care, and tracheal similarity to that of humans.19 In a sheep model, after resection and anastomosis using the annular ligament technique and incorporating adjacent tracheal rings in the sutures, tension of 7.2 to 21.4 N (720 to 2140 g) did not produce tracheal avulsion even though tension-relieving mechanisms were not used.15 A gradual increase in the degree of stenosis was documented. In later studies, this group discovered that an anastomotic site closed with a continuous pattern is nearly as strong as native trachea immediately after surgery and by 1 to 2 weeks is stronger than native trachea, regardless of interrupted versus continuous closure. This suggests that tension-relieving mechanisms may not be necessary.16,18 In support of this statement, an early study of dogs with long segment tracheal resection described that the dogs spontaneously limited neck extension for 7 days after surgery.36 Effect of Age: The adult trachea can withstand forces at the anastomotic site up to about 1700 g, after which results become unpredictable.36,125 This suggests that primary healing occurs up to this point, which corresponds to removal of as much as 50% to 58% of the adult tracheal length. Substantial mobilization of the trachea is required to accomplish this. Although elasticity of the trachea is greatest in young dogs,67 tracheal fragility is increased because tracheal cartilage water content is higher and there is less collagen present.125 In puppies, only 20% to 25% of the trachea can be resected because the juvenile trachea withstands only 60% of the force of the adult trachea.119,125 Anastomoses in young animals present a problem with respect to potential inhibition of tracheal growth. More tracheal narrowing during growth occurs with resection than with simple transection;125 however, puppies reportedly grow without significant stenosis after anastomosis.124 In a rabbit model, monofilament absorbable sutures placed in an interrupted pattern allowed significantly more tracheal growth than did the same suture material placed in a continuous pattern or nonabsorbable suture material in any pattern.132 In puppies, absorbable suture was superior to nonabsorbable in bronchial anastomoses, regardless of the pattern.100 Suture Materials and Pattern: The success of resection and anastomosis depends on early epithelialization of the anastomotic site. In general, the suture type and pattern are of secondary importance, with exact apposition of tracheal ends paramount.14 Monofilament absorbable suture material is preferred because nonabsorbable suture has been associated with increased incidence of granuloma formation and stricture.67,76 Absorbable braided suture material has also been correlated with a higher degree of stricture in sheep.19 Because strength of the sutured trachea exceeds that of native trachea within 2 weeks of surgery, it is not necessary to use material that is slowly absorbed.16,18 Theoretically, advantages of an anastomosis completed with a continuous suture pattern include even distribution of tension across the anastomotic site, reduction of focal ischemia, and an increased tensile strength.16,117 An in vitro study in sheep has proven that continuous anastomoses are stronger than those completed with interrupted suture and even approximate the strength of native trachea.17 However, this phenomenon was not reproducible in vivo,14,16 and in dogs, luminal stenosis was significantly less severe when an interrupted pattern was used.70 It is hypothesized that continuous sutures may have a more pronounced effect on local blood supply, thus delaying healing. In a rabbit model, anastomoses closed with continuous sutures had a significant reduction in local microcirculation versus those closed in an interrupted pattern.170 Similarly, in dogs, microcirculation was found to remain static after tracheal transection but was significantly decreased after continuous anastomosis.169 Behrend and Klempnauer14 also showed that bipolar electrocautery slowed mucosal healing and recommend that hemostasis along tracheal ends is not attempted in any manner. Surgical Options: Multiple means of anastomosis have been described with varying degrees of complexity. Cuffs of mucosa have been preserved, allowing for primary closure of the mucosa alone. Sutures have been placed through individual cartilage rings, around split cartilage rings, and around complete rings through the annular ligament. Overlapping anastomoses have been described, where the smaller end is telescoped into the larger, and step anastomoses have been formed where the ends of the trachea are cut with 90-degree steps that interlock.* A degree of stenosis can be anticipated with all of these techniques; therefore, the “ideal” procedure is one that can be performed reliably and rapidly and is easily reproducible. Hedlund86 described two techniques in dogs and reported success with both end-to-end methods. The “split cartilage” technique involves placing the suture around divided tracheal rings, and the “annular ligament cartilage” technique involves placement of the suture around adjacent tracheal rings through the annular ligament.86,163 Although stenosis was noted in 80% of dogs, all but one were symptom free. The split ring technique caused less stenosis and more precise alignment than the annular ligament cartilage technique.86 It was hypothesized that the split cartilage technique may result in a stronger anastomosis because it heals by fibrocartilage rather than fibrous tissue.88 Both of these techniques were easily and rapidly performed, and no effort was made to preserve a mucosal cuff or avoid the tracheal lumen during the suturing process. As mentioned in earlier studies, the suture material at the time of histopathology was completely encompassed by the tracheal mucosa.36,86,132 The cervical trachea is accessed via a ventral midline approach. This approach can be combined with a proximal sternotomy for lesions at the thoracic inlet. The intrathoracic trachea is typically accessed on the right via a third to fifth lateral thoracotomy.87 The azygos vein is identified, ligated, and transected to yield maximal exposure, and the vagus, phrenic, and recurrent laryngeal nerves are identified and preserved. The area of resection is identified, and stay sutures are placed around cartilage rings proximal and distal to the planned area of resection (Figure 102-13). The trachea is then transected above and below the lesion, either between or through the tracheal rings, and the lesion is removed. With a cervical lesion, the endotracheal tube is advanced into the distal segment. With an intrathoracic lesion, the orotracheal tube is retracted into the cervical trachea. A sterile endotracheal tube is placed by the surgeon into the tracheal distal segment and attached to a sterile circuit to provide adequate oxygenation (Figure 102-14). Tracheal ends are anastomosed with 3-0 or 4-0 monofilament absorbable suture. Sutures are preplaced in the dorsal tracheal membrane first; additional pericartilagenous sutures are placed circumferentially every 2 to 3 mm. For an intrathoracic resection, the final sutures are preplaced at the ventral surface across the gap where the endotracheal tube exits; after placement, the transincisional tube is removed, and the sutures are immediately tied to seal the trachea and permit ventilation. Because leakage of anesthetic gas is expected during intrathoracic tracheal resection and anastomosis, injectable anesthetic should be available or, preferably, the animal should be maintained on intravenous anesthetic and oxygen delivered by an endotracheal tube until the trachea is sealed. Tension-relieving sutures can then be placed around rings proximal and distal to the anastomosis. The thoracic cavity or cervical site should then be flooded with sterile saline and positive-pressure ventilation applied to 20 cm H2O to leak check the site. Closure is routine.
Trachea and Bronchi
Anatomy
Blood Supply, Lymphatics, and Innervation
Physiology
Diagnostics
Computed Tomography
Tracheobronchoscopy
Techniques: General
Indications
Considerations
Technique
Management
Tube Removal
Complications

Permanent Tracheostomy
Technique
Complications
Tracheotomy and Bronchotomy
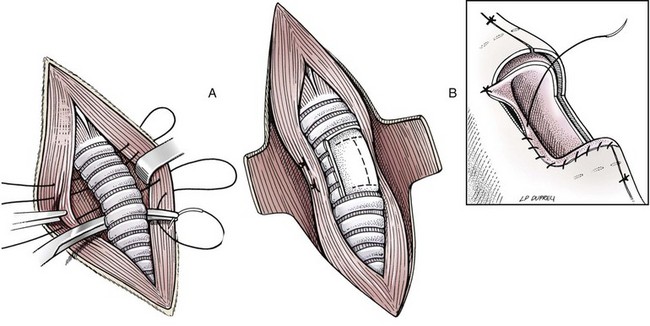
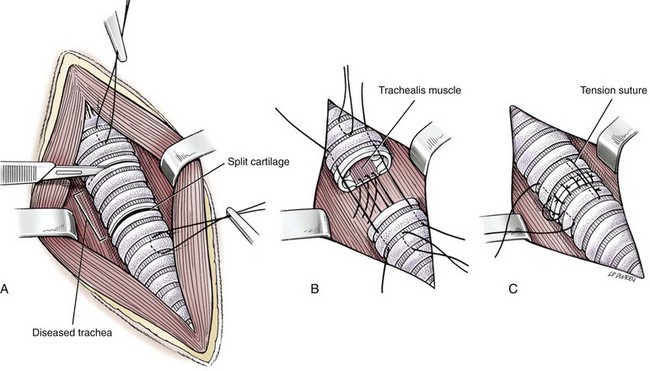
Resection and Anastomosis
Considerations
Surgical Approach
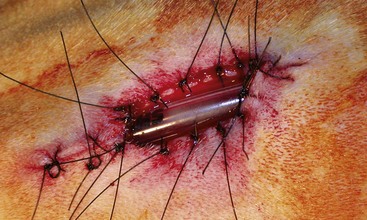
Surgery Technique
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Trachea and Bronchi
Only gold members can continue reading. Log In or Register to continue