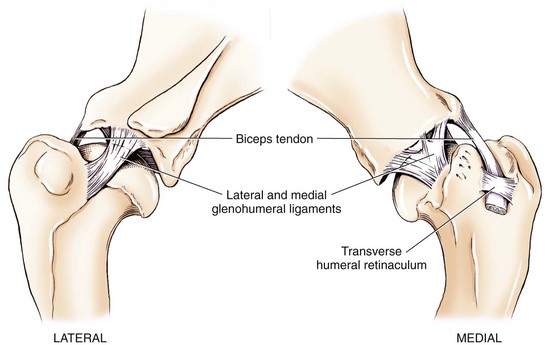
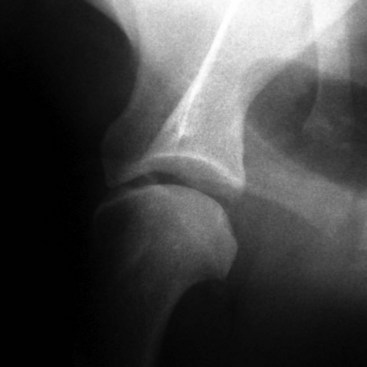
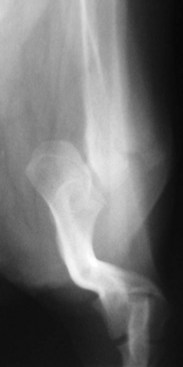
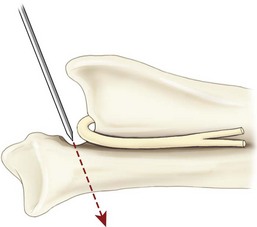
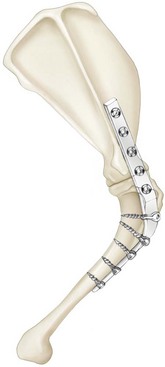
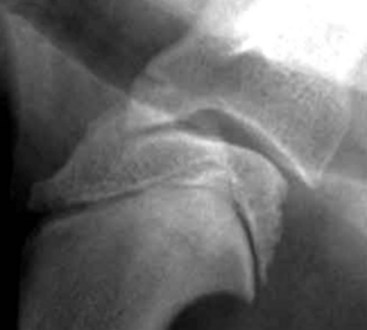
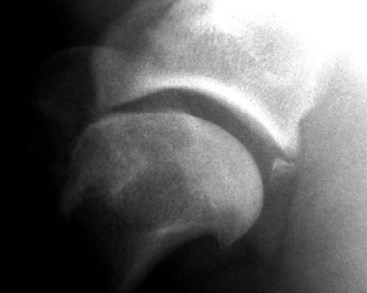
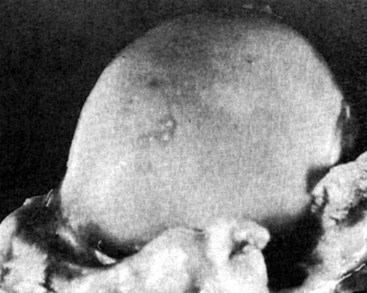
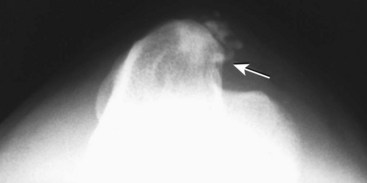
Chapter 51 Coordination of more than 25 muscles is necessary for shoulder motion. Approximately two thirds of the motion of the shoulder region arises from the glenohumeral joint, and one third results from movement of the scapulothoracic synsarcosis. Canine shoulder anatomy has been well described,56 but in brief, the shoulder joint is formed by the articulation of the glenoid cavity of the scapula with the head of the humerus. Both bones have a single center of ossification. Although physeal fusion times vary between bones and sizes of dogs, the glenoid physis is generally reported to fuse within 6 months, and the proximal humeral physis by 1 year of age.24 The glenoid provides relatively little coverage of the humeral head, and the joint, based on topographic distribution of cartilage thickness, is classified as being moderately congruent.71,85 The cartilaginous glenoid lip (labrum glenoidale or labrum) surrounds the glenoid on all sides, is wider on the lateral side, and extends the surface area of the glenoid by 25% to 30%.75 The labrum is triangular in cross-section and is highly vascularized, except along the free margin.75 Both articular surfaces are covered in hyaline cartilage of moderate thickness (approximately 1 millimeter thick in 20 to 25 kg dogs), as is typical of moderately congruent joints.135 The joint capsule attaches proximally at the periphery of the glenoid and distally just distal to the articular portion of the humeral head. Part of the joint capsule surrounds the craniomedial aspect of the tendon of origin of the biceps brachii muscle as it passes through the intertubercular groove. The tendon is held in place by the transverse humeral ligament. The joint capsule is described as blending with the tendons of the “rotator cuff,” which include the subscapularis and coracobrachialis muscles medially, and the supraspinatus, infraspinatus, and teres minor muscles laterally.9,56 Other intra-articular structures of importance are the lateral and medial glenohumeral (collateral) ligaments, which bridge the joint on the lateral and medial aspects, respectively. The medial collateral ligament may be present as a Y-shaped ligament or as a proximocranial-to-distocaudal oblique ligament; the lateral collateral ligament is typically a single large band that tapers slightly as it inserts on the humerus (Figure 51-1). The joint is capable of a wide range of directional movements, including abduction, adduction, and circumduction, but flexion and extension constitute the majority of shoulder movement.69 Normal flexion and extension values for dogs and cats are 57 degrees (flexion) and 165 degrees (extension), and 32 degrees (flexion) and 164 degrees (extension), respectively.62,63 They are best measured by manual goniometry.138 When walking, internal and external rotator angles increase at the beginning and end of the swing phase. Similarly, abduction and adduction angles increase slightly at the beginning of the stance phase and near the end of the swing phase. Flexion and external rotation predominate when the dog is navigating over obstacles.69 Biomechanically, the glenohumeral joint is stabilized by passive (static) and active (dynamic) mechanisms, which function together to provide joint stability through a normal range of motion and serve to counteract forces that would destabilize the joint. Passive stabilizers allow a wide range of joint motion. Joint laxity increases as the joint is flexed, but maximum extension is limited by these static structures.52 Passive mechanisms (those for which muscle activity is not required) include limited joint volume, adhesion/cohesion mechanisms, concavity compression, capsuloligamentous restraints (defined as the glenohumeral ligaments, joint capsule, labrum, and the tendon of origin of the biceps brachii muscle).133 Limited joint volume and adhesion/cohesion principles are interrelated because a small volume of fluid between joint surfaces tends to promote adherence of the two surfaces through the molecular attraction of the fluid to itself and to adjacent cartilage surfaces.52 The stabilizing effect of joint fluid is present when the shoulder joint is in any position; it conserves energy by promoting joint stability without muscle contraction. Concavity compression results from compression of the humeral head into the glenoid cavity. This compression of the convex humeral head into the concave glenoid fossa effectively counters translational forces and stabilizes the joint, but because the humeral head and glenoid surfaces are not exactly congruent, the adhesion–cohesion mechanism may be somewhat compromised.52 A slightly negative intra-articular pressure exists in the normal shoulder joint but is considered to have little functional impact on joint stability during weight bearing.52,132 Active (dynamic) stabilizers of the shoulder joint include the infraspinatus, supraspinatus, subscapularis, and teres minor muscles, and, to a lesser extent, the biceps brachii, long head of the triceps brachii, deltoideus, and teres major muscles.52 Active mechanisms of shoulder joint stability involve the coordinated or selective contraction of the cuff muscles and allow the joint to respond to stresses created by locomotion and weight bearing. Active mechanisms also improve joint stability and glenohumeral balance by enhancing glenoid concavity compression.9,82,87,134,158 These mechanisms are modulated, at least in part, by reflex arcs between nerve fibers in the joint capsule and periarticular muscles.53,71 Reflex arcs serve to promote synergistic activity between ligamentous structures of the joint and associated musculature to maintain joint stability.51 Type 1, 2, and 3 mechanoreceptors present in the collateral ligaments of the shoulder allow the ligaments to serve not only as passive restraints but as sensory structures that actively contribute to joint stability.51 Type 1 (Ruffini) receptors are most common and are more densely concentrated at the cranial aspect of the scapular side of the ligament.51 Immobilization of the shoulder joint results in a marked decrease in passive range of motion, an increase in intra-articular pressure, and synovial hyperplasia and villous proliferation. Although histologic changes occur as early as 4 weeks after immobilization, functional changes do not become apparent until after at least 8 weeks of immobilization and are entirely reversible, even when the period of immobilization is prolonged.132 Lameness is more likely to manifest in dogs with shoulder disease; this condition often is more severe than lameness attributable to other causes of thoracic limb lameness.27 However, diagnosis of the specific cause of shoulder lameness can be elusive and frustrating. Accurate diagnosis should begin with a thorough history of the lameness, including signalment, length of time the lameness has been present, and how the lameness has evolved over time; other historical or concurrent conditions; activity level; previous treatments; and response to those treatments. A general physical examination is important in identifying other problems, especially those that may represent another manifestation of a more generalized disease process that is affecting more than just the shoulder, and those conditions that may create undue anesthetic risk. Observation of the animal walking should be followed by a careful orthopedic examination that includes palpation of the shoulder joint and associated musculature, assessment of the shoulder range of motion and joint stability, and determination of the level of pain associated with the lameness. Goniometric measurements of the normal canine and feline shoulder joint have been validated and can be easily obtained using a manual goniometer.62,63,142 Severe muscle atrophy, loss of conscious proprioception, changes in myotactic and withdrawal reflexes, and axillary masses may suggest a neurologic origin of the lameness and should not be confused with intrinsic orthopedic shoulder disease. When lameness is obvious but cannot be isolated to a particular area, nuclear scintigraphic examination of the limb may reveal the location of the lameness but does not identify the exact cause.133 Arthrocentesis techniques and normal synovial fluid values have been reported for dogs and cats.54,105 Arthrocentesis of the shoulder joint is simple to perform54,123 but requires heavy sedation or anesthesia. Using sterile technique, a 22 gauge needle is directed craniolaterally to caudomedially, entering the joint between the greater tubercle and the acromion process. Synovial fluid analysis provides valuable information for identifying immune-mediated and septic disease, but these conditions are uncommon in the shoulder joint, with most shoulder joint disease conditions being degenerative in nature. Furthermore, synovial fluid parameters can be normal in the presence of intra-articular disease.3 Nevertheless, if radiographic or magnetic resonance imaging (MRI) arthrography is performed, a synovial fluid sample can be obtained at the time of contrast injection. When shoulder lameness is identified or suspected, orthogonal radiographs are generally made. Although radiographs of the shoulder are not reliable for predicting the severity of shoulder joint pathology, radiographic changes, even mild ones, are strong indicators of intra-articular disease. Osteoarthritis is rarely a primary disease of the shoulder joint, so when radiographic signs of osteoarthritis are identified, those changes are usually secondary to some other abnormality.3,7 Similarly, normal shoulder radiographs do not eliminate intra-articular shoulder pathology. Radiographs definitively identify only a few of the many described causes of lameness. Specifically, fractures, osteochondritis dissecans, incomplete ossification of the caudal glenoid, chondrocalcinosis, glenoid dysplasia, and traumatic luxations can generally be identified radiographically. Stress (abduction) views may assist in the diagnosis of medial shoulder joint instability, but the highly mobile nature of the normal shoulder joint warrants caution to avoid overinterpretation of the radiographic angle of abduction of the shoulder joint. Arthrography may be of assistance in outlining soft tissue structures and abnormalities of the shoulder joint, such as the tendon of origin of the biceps brachii muscle, osteochondritis dissecans flaps, medial joint stabilizers, intra-articular loose bodies, and synovial neoplasms and other disorders.25,141,153 Any iodinated contrast agent that is safe for intravenous administration (e.g., meglumine-sodium diatrizoate, sodium/meglumine amidotrizoate) can be used, but newer, dimeric nonionic compounds such as iotrolan or iohexol are preferred.108,153 Meglumine-sodium diatrizoate, an older contrast agent, has been used traditionally for arthrography but required dilution, created slightly more synovial inflammation than newer agents, and tended to rapidly dissipate, making completion of diagnostic studies difficult. Some authors recommended adding 0.2 mg epinephrine to the meglumine sodium diatrizoate prior to injection to delay synovial absorption of the agent.148 Concerns for rapid resorption of contrast agent do not arise when iohexol is used, and the addition of epinephrine, although seemingly safe, is unnecessary.108 Iohexol, unlike older agents, does not require dilution unless the joint is to be imaged with computed tomography (CT). When CT is used, iohexol and other, similar agents should be diluted 50% in sterile water or saline.108 The increasing availability of noninvasive imaging techniques such as MRI and ultrasonography has reduced the use of arthrography in veterinary practice, but it remains a useful tool when other options are not available. Ultrasonographic evaluation of the shoulder is most accurately performed using a 7.5 to 10 MHz, linear, phased array transducer. This transducer offers the best near field resolution and the least distortion.84 Alternatively, a standoff pad can be included if less capable transducers are used. Smaller probe sizes facilitate positioning of the probe along the medial and craniomedial side of the joint when the tendon of origin of the biceps brachii muscle is imaged.84 Ultrasonography using high-frequency linear transducers can dependably identify the tendon and its associated structures. Ultrasonographic examination of the tendon also facilitates safe, efficient aspiration of surrounding synovial fluid and therapeutic injections. The tendons of the supraspinatus, infraspinatus, and teres minor muscles and the caudal aspect of the humeral head can also be reliably imaged ultrasonographically.74,84 Unfortunately, one current limitation of ultrasound examination of the shoulder is the inability to properly visualize the medial joint structures, where pathology is common.84 MRI is the most recent modality to become available for clinical evaluation of the shoulder. MRI of the canine shoulder has been described in both normal and disease states.2,96,129,150 Although MRI offers significant improvement over other imaging modalities in revealing changes in soft tissue structures, overinterpretation and underinterpretation do occur.96 Sagittal, transverse, and dorsal plane proton density fat saturation and T2-weighted gradient echo sequences should be obtained to fully evaluate the shoulder; MRI arthrography (using gadopentetate dimeglumine) does not improve the quality of those studies.2,128,152 An advantage of MRI over ultrasonography is the ability of MRI to evaluate all periarticular soft tissue structures, whereas ultrasonographic examination of the shoulder is limited to the cranial, lateral, and caudal aspects of the shoulder. Unlike arthroscopy, MRI also allows identification of both intra-articular and extra-articular structures of the joint.96 As much as 15% of shoulder pathology identified in one study was considered extra-articular and would have been missed with arthroscopic examination alone.96 More accurate assessment of MRI images will likely become commonplace as the availability of MRI increases and experience with the technology grows. Osteochondrosis is a disturbance of the normal process of endochondral ossification. When osteochondrosis progresses to the formation of a cartilaginous flap, the term osteochondritis dissecans is used. The pathophysiology of osteochondritis dissecans was described first in 1956 and then in the English-speaking literature by Mostowsky in 1966.79 The pathogenesis, clinical signs, and management of osteochondritis dissecans have been well described in the literature79,103,111,143 and are discussed in detail in Chapter 73. The caudocentral or caudomedial aspect of the humeral head, usually opposite the caudoventral rim of the glenoid, is the most common site of the cartilage flap identified with osteochondritis dissecans of the shoulder joint. The cartilage in this area is normally relatively thinner than cartilage at the periphery of the humeral head,73,168 so increased cartilage thickness in the caudocentral aspect of the humeral head as observed with osteochondritis dissecans occurs as a result of the disease. Why increased cartilage thickness and resulting fragmentation of the cartilage consistently occur in this region of the humeral head remains unknown but may be related to the arrangement of the trabecular bone and increased hydraulic resistance and flexibility of the humeral head when compared with the glenoid fossa.135–137 This area of the humeral head is also an area of primary load transfer across the joint to the corresponding glenoid72,102; an early paper suggested that osteochondritis dissecans may be a manifestation of trauma.79 Large or giant-breed male dogs are most commonly affected, and 27% to 68% of dogs have bilateral lesions.14,102,113 A single report of a cat with osteochondritis dissecans of the humeral head has been recorded.109 Affected dogs are often consuming a high-protein, high–caloric content diet, typical of so-called “puppy diets.” Excessive protein, calories, and calcium have been implicated in the pathogenesis of osteochondritis dissecans, but debate continues regarding their respective contributions to the development of the disease.98,120 Although bilateral lesions are often present radiographically, the lameness associated with osteochondritis dissecans is usually unilateral and typical of dogs with osteoarthritis in that it worsens with time and is accentuated by rest and exercise. Clinical signs generally develop between 4 and 8 months of age. Atrophy of the supraspinatus, infraspinatus, and deltoideus muscles may be present if the lameness is chronic. Pain with palpation and detection of crepitus are uncommon, but passing the joint through a range of motion, especially extreme extension, often elicits a pain response. Although the primary lesion of osteochondritis dissecans always occurs while the animal is growing, some dogs do not demonstrate clinical signs when young, instead developing clinical signs later in life, usually during middle age, as the result of secondary osteoarthritis. Diagnosis of osteochondritis dissecans of the humeral head is usually made by radiographic examination of the shoulder joint. The earliest radiographic changes seen with osteochondrosis include loss of trabecular structure and subchondral lysis.70 These changes generally are not observed because dogs with osteochondrosis remain sound until a cartilage flap is formed, at which time radiographic examination reveals a subchondral bone defect in the caudal aspect of the humeral head on the mediolateral view70 (Figure 51-2). If no defect is observed, a more medial lesion may be present, or radiographic positioning may obscure the lesion. The most common radiographic technical errors that prevent identification of the subchondral bone defect include poor radiographic detail, oblique positioning of the shoulder, and failure to adequately distract the limb away from the thorax. A mediolateral radiographic view with the humerus slightly internally or externally rotated may demonstrate the lesion if the lesion is located, respectively, on the caudomedial or caudolateral humeral head.70,114 Gas accumulation in the joint space (vacuum phenomenon, or cavitation) may be associated with osteochondritis dissecans149 but is not pathognomonic for osteochondritis dissecans. Mineralized loose bodies in the caudal joint pouch or in the tendon sheath of the biceps brachii muscle may be observed. A craniocaudal projection may assist in identification of loose bodies in the bicipital tendon sheath.70 If osteoarthritis is recognized in a mature patient and is suspected to be secondary to osteochondritis dissecans, a subchondral defect of the caudal humeral head should be present. Because osteochondrosis often is present bilaterally (approximately 50% of dogs with shoulder osteochondritis dissecans)65 but is manifest only unilaterally as osteochondritis dissecans, the recommendation is often made to radiograph both shoulders at the time of diagnosis of unilateral osteochondritis dissecans. The author’s alternative opinion is that, if clinical signs are identified unilaterally, radiographing both shoulders is not efficacious because, even if a lesion is identified in the unaffected shoulder, osteochondrosis does not necessarily advance to osteochondritis dissecans. However, the owner should be counseled that osteochondrosis may be present in the sound leg and may progress to osteochondritis dissecans, and that a definitive diagnosis can be pursued at such time as lameness occurs. Use of positive-contrast arthrography, MRI, and ultrasonography has been described for the diagnosis of osteochondrosis.147,148,151,155 In the author’s opinion, little is to be gained over conventional radiography by the use of these imaging modalities, except for the identification of nonmineralized cartilage flaps trapped within the bicipital tendon sheath (occurs in approximately 10% of shoulder osteochondritis dissecans cases145). Finally, synovial fluid analysis may reveal changes typical of degenerative joint disease but is not diagnostic for osteochondritis dissecans. It is generally accepted that as long as the cartilage flap remains attached to the humeral head, pain and lameness will persist.65 Medical therapy, although universally agreed to be inferior to surgical removal of the osteochondritis dissecans flap, involves vigorously exercising the dog in an attempt to break loose the cartilage flap.102 Once the flap breaks loose, pain generally resolves unless the flap migrates into the bicipital tendon sheath. Surgical removal of the cartilage flap associated with osteochondritis dissecans is the best course of action for resolving joint pain and achieving optimal return to function.65 Arthroscopy provides a minimally invasive way to confirm the diagnosis and treat the disease simultaneously and allows the surgeon to evaluate areas of the joint that cannot be viewed by arthrotomy. The flap is observed through a lateral portal, and a caudolateral instrument portal is created to remove the flap. The remaining subchondral defect is debrided to bleeding subchondral bone using a bone curette or a motorized shaver. When possible, avoiding beveling the edges of the defect has been recommended to decrease the risk of fibrillation and erosion of the cartilage of the corresponding surface of the glenoid cavity.126 The cause of degenerative changes in the glenoid cartilage is unknown, but in experimentally created osteochondral lesions, they are presumed to be the result of loss of contact with the cartilage of the humeral head created by the larger-diameter defect created by beveling. Anecdotally, larger osteochondritis dissecans lesions have been correlated with poorer clinical outcomes, so the concept of limiting the size of the defect to the dimensions of the original flap seems prudent. The same techniques of flap removal and treatment of the subchondral bed can be performed through an arthrotomy. Several surgical approaches are suitable for removal of an osteochondritis dissecans flap.110 Depending on surgeon experience, assistance, and equipment, the caudal approach, as described by Gahring, or the craniolateral approach, as described by Hohn, probably offers the best view of the caudal humeral head.110,115 The caudal approach requires a surgical assistant to provide tissue retraction for adequate viewing of the osteochondritis dissecans flap but results in less loss of shoulder range of motion and increased weight bearing (as evidenced by increased peak vertical forces when compared with the lateral approach), at least within the first month after surgery.90 The lateral approach offers increased exposure of the caudal humeral head.90 Regardless of how the osteochondritis dissecans flap is removed, the prognosis for minimal osteoarthritic change and normal or near normal function after treatment in the long term is excellent. Rehabilitation exercises should be performed following surgery to maximize the speed and extent of recovery.86,127 Hypoplasia or dysplasia of the glenoid can result in a grossly abnormal articulation of the shoulder joint, leading to medial luxation of the shoulder.22,162 The cause of the abnormal development is unknown. Congenital luxation is generally diagnosed in dogs between 3 and 10 months of age.21 The condition is usually unilateral.162 Breeds diagnosed with glenoid dysplasia include Toy and Miniature Poodle, Chihuahua, Pomeranian, Shetland Sheepdog, Norwegian Elkhound, Collie, Dachshund, Pug, Pekingese, Miniature Pinscher, Lhasa Apso, Griffon, Wire-Haired Fox Terrier, and Cavalier King Charles Spaniel.161 Often a history of no or only mild trauma is reported. Animals with medial luxations may hold the affected limb with the elbow flexed and adducted and with the distal limb abducted. Abnormal spatial differences between the acromion and the greater tubercle are appreciated with palpation. Mediolateral and craniocaudal radiographs of the shoulder reveal a misshapen glenoid cavity and, less commonly, a flattened humeral head (Figure 51-3). Medical management of glenoid dysplasia is not recommended.162 Excision arthroplasty of the glenoid, with or without excision of the humeral head, is the preferred treatment.19,45,111 Arthrodesis can be done as an alternative to excision.156 Excision Arthroplasty.: The intent of excision arthroplasty is to form a “false” or fibrous joint between the humeral head and the scapular neck, while removing the articular structures that contribute to the development of osteoarthritis and resulting pain. A lateral approach111 to the shoulder is recommended, and the glenoid is excised by making a distolateral-to-proximomedial osteotomy of the scapular neck with an osteotome or, preferably, a sagittal saw (Figure 51-4). The suprascapular nerve should be identified and protected while the osteotomy is performed. Although the procedure is usually performed in small-breed dogs, good to excellent function, no pain with palpation, and no to moderate humeral muscle atrophy have been reported in a limited number of cases.45 Some authors recommend concurrent excision of the humeral head, but little evidence supports recommendation of this additional step.19 Intuitively, physical therapy after surgery should improve functional outcomes, but no definitive research is available to confirm such a supposition. Arthrodesis.: Arthrodesis is an alternative to glenoid or humeral head excision arthroplasty but is technically demanding, is potentially prone to implant failure and delayed union, results in loss of motion in the joint, and has not been shown to result in better functional outcomes compared with excision arthroplasty. Subjective evaluation of a very limited number of cases (16) in a range of sizes of dogs suggests that good to excellent outcomes generally are obtained following arthrodesis.39,117,156 In small dogs, arthrodesis can be performed by placing a large transarticular screw or diverging Kirschner wires (with or without tension band wire); this method is not recommended in medium- or large-breed dogs, and clinical outcomes are generally better when plates and screws are used for all sizes of dogs and cats. If, as in most instances of glenoid dysplasia, the glenoid cavity is grossly misshapen, an osteotomy of the distal tip of the glenoid and corresponding humeral head may be required to create an acceptable degree of contact between the two bone surfaces. Caution must be exercised to osteotomize the two surfaces in such a way as to achieve maximum contact and an acceptable joint angle, while avoiding creating malangulation of the joint in the mediolateral and craniocaudal planes. Cancellous allograft or autograft (although augmentation with cancellous bone graft is perhaps not as critical in this joint because of the normally high density of cancellous bone in the humeral head) can be placed in the articular space to facilitate bony union. A transarticular Kirschner wire or a small Steinmann pin can be placed to temporarily maintain the joint in its proper position, while the bone plate and screws are applied along the cranial aspect of the humerus and the craniolateral aspect of the scapula, where the spine fuses with the body (Figure 51-5). The plate should extend from the distal half of the scapula to the proximal half of the humerus (typically at least four or five screws in each bone). The plate is contoured to achieve a shoulder angle of approximately 105 to 110 degrees, while avoiding humeral varus or valgus. Adjustments to the angle of arthrodesis should be made if examination of the animal’s opposite shoulder angle while weight bearing or other factors suggest that 105 to 110 degrees is excessive or insufficient.164 Some torsion of the proximal plate is usually required to achieve proper contouring to the scapula. Caution should be exercised to avoid internal or external rotation (the greater tubercle should be positioned craniodistal to the acromion and slightly medial in the sagittal plane when the shoulder is in a rotationally neutral position). Locking plate technology may be preferred for shoulder arthrodesis because the relatively thin bone of the scapula makes screw pull-out and construct failure more likely when conventional plates and screws are used. If osteotomies of the greater tubercle or acromion were performed, the bone is reattached as near to the original anatomic site as possible using a pin and tension band or lag screws. If tendon transection was required, the tendon of the infraspinatus muscle is reattached to its insertion or to the adjacent tendon of the teres minor muscle. Multiple epiphyseal dysplasia is a rare, presumably autosomal recessive genetic disease characterized by a defect in ossification of the epiphyses of long bones, vertebrae, cuboidal bones, and apophyses.125 Radiographic demonstration of epiphyseal dysplasia is evident by 8 weeks of age, and severe lameness generally appears by 5 to 8 months of age (Figure 51-6). Multiple epiphyseal dysplasia has been reported in a variety of medium- and large-breed dogs. The epiphyseal dysplasia resembles bony changes observed in congenital hypothyroidism but is a distinctly different entity. The prognosis for pain-free function is poor, and euthanasia is generally recommended.125 Hypertrophic osteodystrophy (HOD) is a developmental disease of unknown origin that usually affects skeletally immature large- and giant-breed dogs. Lameness and metaphyseal swelling are accompanied by general malaise and pyrexia in many dogs. The radiographic hallmark of hypertrophic osteodystrophy is an irregular radiolucent line in the metaphysis parallel and separate from the physis. Hypertrophic osteodystrophy has been well described in other references and is included here only because it has been recently reported in the proximal humerus.46 Incomplete ossification of the caudal glenoid has been reported as a cause of shoulder-related lameness.124,143 Incomplete ossification of the caudal glenoid has been reported as a cause of lameness in medium- to large-breed dogs ranging from 8 months to 10 years of age.102,143 Reports as early as 1974 show radiographs demonstrating what were termed ossicles that resemble incomplete ossification of the caudal glenoid but were considered manifestations of osteochondritis dissecans.143 Incomplete ossification of the caudal glenoid is usually asymptomatic, but pain may result if the caudal fragment is mobile. Mild to moderate shoulder pain and regional muscle atrophy are usually present. The incompletely ossified caudal glenoid is easily demonstrated by a mediolateral radiograph (Figure 51-7). Arthroscopic examination typically reveals an osteochondral fragment with variable degrees of villous synovial hyperplasia. Pathologic findings consistent with other diseases of the shoulder joint may also be present, so a thorough examination of all intra-articular structures is important. If it is mobile, the fragment should be removed when manipulated with an arthroscopic probe.102 In the small number of reported cases, arthroscopic removal of the fragment and debridement of the adjacent edge of the glenoid resulted in resolution of lameness.102 Chondrocalcinosis, sometimes referred to as pseudogout, is a rare disorder in the dog. Chondrocalcinosis consists of deposition of hydroxyapatite in the articular cartilage. The lesion is found primarily in the plateau region of the humeral head of Greyhounds, and it has also been reported in the femoral head of German Shepherd Dogs.121 Lesions may occur as a single spot or as small, multifocal areas of chondrocyte mineralization. Histologically, articular surface lesions appear as a white, raised focus of mineralized chondrocytes surrounded by a translucent halo (Figure 51-8).167 Grossly, small pits in the cartilage surface, scarring, scoring, and cracking of the articular surface are also reported.167 The lesions are typically unilateral but can be present bilaterally. Bilateral lesions in racing Greyhounds tend to be more pronounced in the right shoulder because of the repetitive high pressure placed on that joint during Greyhound racing.121,167 This shoulder lesion is often found incidentally during necropsy and without clinical signs. Its clinical relevance is unknown. Disease of the tendon of origin of the biceps brachii muscle was originally labeled tenosynovitis but, over time, has become more commonly described as a tendinopathy. Bicipital tendinopathy is considered by some authors to be the most common cause of shoulder lameness in dogs,7 but its true prevalence is unknown. Lameness previously attributed to bicipital tendinopathy is now being identified as originating from other soft tissue structures associated with the shoulder. This refinement is due largely to the capabilities of cross-sectional imaging modalities such as ultrasound and MRI to identify soft tissue injury and disease.11,41,84,97 Bicipital tendinopathy is almost exclusively a disease of dogs but has been reported in conjunction with glenoid dysplasia in a cat.130 Bicipital tendinopathy in dogs has been classified as primary or secondary.49 Primary bicipital tendinopathy is thought to result from inflammation of the tendon of origin of the biceps brachii muscle as a result of overuse or chronic repetitive injury. The region of the tendon near its origin on the supraglenoid tubercle is an area of relative hypovascularity, which may dispose the tendon to mechanical failure (fraying or rupture).7 Secondary bicipital tendinopathy may occur in response to other intra-articular disease or cartilaginous loose body entrapment beneath the tendon, or as the result of acute trauma to other tendons associated with the shoulder.49,140 In either case, bicipital tendinopathy historically has been assumed to occur as a result of the inflammatory process that follows the underlying cause. Recent studies in human beings have shown that many cases of tendinopathy may not be inflammatory or due to overuse.49 Similarly, several reports in the veterinary literature document the lack of inflammatory changes in some instances of bicipital tendinopathy. Therefore, although the histopathologic lesions of tendinopathy and an arthroscopic classification of biceps tendon lesions have been described in dogs,11,34,49 the exact pathophysiologic mechanisms responsible for these changes remain unknown. History.: Dogs affected by bicipital tendinopathy are usually middle-aged to older, medium- to large-breed dogs. No breed or sex predilection has been noted. Clinical signs are variable but often include a chronic intermittent or progressive weight-bearing thoracic limb lameness, which becomes worse with exercise.163 Lameness is often refractory to oral administration of antiinflammatory drugs. Diagnosis of bicipital tendinopathy can be made by using some combination of the shoulder drawer test, direct palpation of the tendon of origin of the biceps brachii muscle, the biceps retraction test, radiography, contrast arthrography, ultrasonography, MRI, and arthroscopy. Physical Examination Techniques.: The nomenclature for physical examination tests to evaluate the tendon of origin of the biceps brachii muscle differ between English and German literature. Palpation of the tendon in the intertubercular groove while flexing the shoulder is called the biceps tendon test in the English-speaking literature. The test is performed by placing and maintaining direct digital pressure against the tendon of origin while the shoulder is flexed and the elbow extended. A noticeable pain response is considered positive for bicipital tendinopathy by most165 but has been suggested by others to be more indicative of generalized shoulder pain.9,140 In the German literature, the same test name refers to full extension of the elbow while the shoulder is completely flexed, indicating a ruptured tendon—a much less common condition.165 Another test that can be useful for diagnosing bicipital tendinopathy is the drawer test. This test does not evaluate the physical integrity of the tendon of origin of the biceps brachii muscle (i.e., it is not like the cranial drawer test used to diagnose rupture of the cranial cruciate ligament) but rather places direct pressure against the tendon and the associated synovial sheath. In performing this test, the shoulder is partially flexed, the scapula stabilized with one hand, and the humeral shaft grasped with the opposite hand. The humerus is pushed cranially, placing pressure against the biceps tendon. If the tendon is diseased (and the associated synovium inflamed), a marked pain response occurs.9 A third test that has been described for diagnosing bicipital tendinopathy is the biceps retraction test. This test is performed by grasping the tendon of insertion of the biceps brachii muscle near the cranial aspect of the elbow and pulling caudally with the dog in a weight-bearing stance. A painful response is considered positive for bicipital tendinopathy.18 Diagnostic Imaging.: When bicipital tendinopathy is suspected, standard orthogonal radiographic views of the shoulder are made. Because the radiographic changes associated with bicipital tendinopathy may be subtle, a flexed craniodistal-cranioproximal (skyline) view may be useful for identifying irregularities in the intertubercular groove and mineralization of the tendon112,140,145 (Figure 51-9). The skyline view is also helpful for separating mineralization in the tendon of origin of the biceps brachii muscle from mineralization in the tendon of the supraspinatus muscle, because the two tendons are somewhat superimposed when viewed in the lateral radiographic projection. Contrast arthrography can be valuable for identifying irregularities associated with the tendon and the surrounding synovium and is considered by some to be more sensitive than ultrasonography for diagnosis.74,122,140
The Shoulder
Anatomy
Biomechanical Physiology
Diagnosis of Shoulder Disorders
Arthrocentesis
Radiography
Cross-Sectional Imaging
Conditions Affecting the Bones of the Shoulder
Diagnosis
Treatment
Glenoid Dysplasia
Treatment
Multiple Epiphyseal Dysplasia
Hypertrophic Osteodystrophy
Incomplete Ossification of the Caudal Glenoid
Chondrocalcinosis
Conditions Affecting the Soft Tissues of the Shoulder
Diagnosis
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


The Shoulder
Only gold members can continue reading. Log In or Register to continue