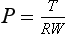Chapter 19 In modern surgery, we are well aware that surgical technique has an effect on patient survival and postoperative outcome. Good surgical technique was defined by Halsted (see Chapter 18) and includes the principle of accurate hemostasis. As surgeries become more invasive and complex, strategies for adequate hemostasis become more important to achieve shorter surgery times, reduced postoperative morbidity and mortality, and lower reoperation rates. In veterinary surgery, whole blood and blood products generally are in short supply and are not readily available, so it is important for both biologic and financial reasons that blood products are not used to compensate for poor hemostasis. The concept of actively trying to stop bleeding is relatively recent, despite evidence that even ancient societies knew that uncontrolled bleeding could be fatal. The earliest evidence of thermal cautery has been found on Neolithic skulls, and Egyptian scrolls describe using heat to reduce bleeding. The use of extract of the plant ephedron as an arterial constrictor is described by Pliny the Elder in 79 AD and may have been used in China as early as 5000 BC, but it was not introduced into Western Medicine until 1924. In Europe, the earliest reports of active hemostasis used tourniquets, unfortunately applied to the nonbleeding limbs (270 BC), and this mistake was not corrected until the first tourniquet as we know it was reported in 1718 AD, with the Esmarch tourniquet described in 1873 AD. The science of patching up gladiators in Roman times was well recorded (150 AD), and physicians in charge of the most valuable gladiators were aware that elevation of the bleeding limb reduced blood flow, and had tried twisting bleeding arteries with hooks, as well as ligating them with silk.51 In modern surgery, control of bleeding in the surgical patient is important for a number of reasons. Hemostasis achieves a “dry” surgical field, thereby improving visibility and reducing the risk of iatrogenic injury. Tissue healing is improved, and postoperative edema and risk of infection are reduced. In addition, postoperative morbidity is reduced as a result of decreased blood loss or hypotension. In surgery on human beings, well-established postoperative morbidity and mortality are associated with multiple blood transfusions, and improved hemostasis will have an impact on this.59,69 During the process of surgery, injured vessels trigger the normal physiologic response: contraction of the vessel wall, formation of a platelet plug, and local activation of the cascade at the point of injury. These small individual injuries may not be significant on their own, but the cumulative effect of these small injuries can be substantial. In complex or long surgeries, the normal physiologic response to bleeding may not be sufficient to achieve complete cessation of all bleeding points before systemic consequences are seen. As the surgery progresses, the small amount of blood lost from each bleeding vessel before it seals becomes increasingly significant. Eventually, consumption of clotting factors occurs, and the clotting process itself becomes less efficient, contributing to morbidity associated with blood loss. Bleeding during surgery can be minimized by using cutting techniques that seal these small vessels as they are cut, for example, the laser scalpel, electrosurgery, and Ligasure (Covidien, Waltham, MA; see Chapter 16). However, in some circumstances, bleeding occurs from multiple small vessels or vessels that are difficult to access, and bleeding is difficult to localize or control using these cutting techniques. Examples of such circumstances include surgery involving the nasal turbinates, friable surfaces (liver), bone surfaces, or abraded skin/wound surfaces, or circumstances where the tissues are delicate and the method used must not cause delayed healing or damage to adjacent structures. When such circumstances are encountered, other techniques are used to augment hemostasis. Three main principles that can be used to augment hemostasis include the following: The principle is to slow blood flow into the area, thus encouraging and allowing clot formation and stabilization. It takes about 30 seconds for platelet aggregation into a soft clot and a further 2 to 3 minutes for this clot to be cross-linked with the formation of a fibrin matrix that provides some security and seal of the vessel defect (see Chapter 7). A traditional first aid approach is to apply pressure to a bleeding vessel. This apposes the torn edges of the vessel wall and allows time for the platelet plug to form and stabilize. Minor hemorrhage may cease with 2 to 3 minutes of gentle pressure with a surgical sponge and not require further treatment; however, care should be taken when the sponge is removed that the fragile clot is not dislodged.82 The duration of pressure that is necessary for coagulation is dependent on multiple factors, including vessel diameter, blood pressure, and coagulation ability. For severe bleeds, this is a temporary measure to decrease active hemorrhage and allow definitive treatment of the source of hemorrhage. Abdominal hemorrhage has been treated using tamponade with abdominal counterpressure bandages, and this has been reported to improve survival in dogs with experimental hemoperitoneum, although this has not been reported in clinical veterinary patients.53 The duration of counterpressure application should be cautious, with close monitoring of urinary output used to check for abdominal hypertension. In human beings, raised intrathoracic and intracranial pressures have been reported with abdominal counterpressure, and it would seem logical to use this technique with caution in patients with concurrent thoracic or cranial injury.29 Pressure can also be applied in the abdomen by packing with surgical towels and temporarily closing the abdomen. This allows stabilization of the patient for 24 to 48 hours before reoperation and removal of the packing.29 Topical vasoconstrictors, such as epinephrine, adrenaline, or ephedrine, have been used for centuries,51 but in modern veterinary medicine they usually are used in the management of diffuse inaccessible bleeding such as epistaxis or small arterial bleeding in routine procedures such as onychectomy. Topical endoscopic adrenaline injections have also been reported to treat bleeding gastric ulcers.38,49 Disadvantages include the absorption of active agents that have effects on the cardiovascular system and profound vasoconstriction locally at the site of administration, or systemic responses resulting in generalized peripheral vasoconstriction that may cause ischemic damage. It is generally recommended that adrenaline is used in a diluted form, but sources are contradictory as to what the dilution should be, ranging from 1:1000 to 1:100,000 U/mL.49,76,97 Blood flow to the surgical area may be controlled by using pressure to occlude arterial and venous flow, and in some circumstances, it is possible to temporarily occlude the arterial supply to prevent hemorrhage using vessel tourniquets. Selective arterial/venous temporary compression or ligation can be achieved by using vascular clamps (bulldog or Satinsky), snares, or the surgical assistant’s fingers (Table 19-1, Box 19-1, and Box 19-2). Table • 19-1 Arteries/Veins That Can Be Occluded on a Temporary Basis to Assist Surgery Rumel’s tourniquet is used frequently in vascular and cardiothoracic and minimally invasive surgery to temporarily control blood flow through vessels during surgery.29,91 Umbilical tape is applied around the vessel with the ends passed up through tubing and secured tightly using a clamp on the end of the tapes at the top of the tubing. The tourniquet can be loosened by releasing the tape ends slightly and can be easily retightened by pulling them back up the tube. Tourniquets: Control of blood flow to the extremities is often used to improve surgical visibility or to control traumatic bleeding. Tourniquets are applied at the proximal part of the limb to occlude blood flow to the distal limb, and it is generally accepted that in the emergency situation, more lives are saved by tourniquet use than limbs are lost, although this has not been documented in veterinary studies.45 Conventional tourniquets are broad, tight bandages applied to the proximal aspect of a limb to prevent arterial and venous blood flow. Before tourniquet application, the limb is often elevated to provide partial exsanguination and to prevent blood from pooling within the limb, thereby further minimizing blood loss. Esmarch tourniquets are usually made of a broad elastic material and are applied tightly from distal to proximal, thus exsanguinating the limb.22 The Esmarch bandage is then secured tightly at the proximal aspect of the limb, preventing blood from flowing into the limb during surgery; thus the limb is not only prevented from bleeding but also has had the blood removed. The advantages of this are clear in terms of a bloodless surgery; however, it may be difficult to identify when large vessels are punctured and require ligation, and it is clearly a very ischemic event for the tissues of the limb. Application of tourniquets is not without risk; in human beings, systemic responses to tourniquets are well characterized, and increases in circulating blood volume, hypertension, and hypercoagulopathy when the tourniquet is applied have been recorded. After removal of the tourniquet, there may be transient, but marked, hypotension, hypercapnia, and increased cerebral blood flow and intracranial pressure (see Chapter 38); pulmonary thromboembolism (and death); and increased fibrinolytic activity, which can cause increased bleeding.8,9,18,57 Reperfusion after tourniquet removal may also cause tissue edema due to reactive hyperemia, and prolonged tourniquet use will result in ischemic and direct tissue damage, which may be worse in tissue that is already damaged through trauma or surgery.5,8,58 In human beings, a well-recognized syndrome of nerve damage and pain is associated with tourniquet use. This syndrome is characterized by increased blood pressure during the procedure, along with postoperative palsy or paralysis.8,18,45,56,72 The site of tourniquet application is important, as direct pressure can potentially cause muscle, nerve, or skin necrosis.5,8,64,65 Tourniquet use in human beings for joint replacement and for tendon, nerve, and vessel repair is well established.18 Postoperative intramuscular swelling in human beings can last for up to 1 to 6 weeks, with pain, weakness, and edema reported. Tourniquets initially result in a physiologic nerve block due to ischemia, but high tourniquet pressures (>1000 mm Hg) can result in degeneration of the myelin of compressed nerves, causing neurologic deficits for up to 6 months postoperatively.34,62 It is now considered best practice to use pneumatic tourniquets for surgery on human patients.37 These deliver a controlled pressure to the limb at the level of the tourniquet and reduce the incidence of complications.18 The occlusion time is measured, as well as the pressure. The cuff pressure is set at a calculated pressure for the duration of surgery; generally this is recommended to be approximately 100 mm Hg above the patient’s systolic pressure. Another option is to use a Doppler sensor that enables the anesthetist to determine the minimum tourniquet pressure needed to occlude the arterial supply and thereby use the lowest effective tourniquet pressure, which may cause less damage to the muscle directly underneath the tourniquet. In the absence of a direct means of measuring pressure applied by the tourniquet, the tourniquet pressure (force per unit area) can be calculated using the following formula, which shows an inverse relationship between the pressure applied and the width of the tourniquet itself85: where P = pressure (Pa), T = bandage tension (N), R = radius of curvature of limb (m), and W = bandage width (m). Recommendations on the use of surgical tourniquets in veterinary surgery are unsupported by clinical data; clinical practice therefore should be conservative, in keeping with available information. The duration of safe ischemia for the limb in dogs or cats has not been established. Experimental studies on muscle ischemia in healthy dogs showed that the tissue was not irreversibly damaged for several hours (4 to 24 hours).40,77,96 However, these studies do not state the pressure gradient between systolic patient pressure and the tourniquet, and other studies demonstrate that energy stores are depleted within 2 to 3 hours, and mitochondrial changes are visible after 1 hour, with microvascular damage evident after 2 hours.18 It would therefore seem appropriate to assume that all tourniquets should be applied for the shortest possible time, and most recommendations suggest a maximum time of 1.5 to 2 hours, which is thought to correspond to the point at which muscle adenosine triphosphate (ATP) stores are depleted. At this point, the tourniquet may be released for 10 to 15 minutes, with the limb allowed to exsanguinate by elevation, and then may be reapplied. However, reapplication increases the risk of both systemic and limb complications due to reperfusion and prolonged ischemic times. The tourniquet time should be reduced in circumstances where tissue trauma is significant, or where sepsis is the primary injury.18 The limb should be elevated before tourniquet application to allow exsanguination, and the widest cuff practical should be used to apply pressure at the widest part of the limb. However, exsanguination of the limb before tourniquet application is not recommended in the presence of malignant neoplasia or infection.8 The time of application should be recorded and cardiovascular parameters monitored. Where possible, the pressure should be adjusted according to the patient’s systolic pressure. Where antibiotics are indicated, it is logical to administer them 20 minutes before application of the tourniquet, although one clinical trial found no benefit with this regimen.21,63,68,81 Finally, some experimental studies have demonstrated that local hypothermia of the limb before and during tourniquet application may reduce ischemic damage, thereby allowing longer tourniquet times safely, but this has not been investigated clinically.79,84 Postoperative care of the limb is also important. Vascular permeability may be increased in the limb as the result of hypoxic reflexes and reperfusion responses, and the risk of postoperative edema or swelling in the limb is increased. Where casting or bandaging is indicated postoperatively, a risk of bandage injury is present in the 24 hours following tourniquet application. 5,8,18
Surgical Hemostasis
Hemostatic Agents
Blood Flow Reduction
Pressure/Tamponade
Topical Vasoconstrictors: Epinephrine/Adrenaline/Ephedrine
Distant Control of Blood Flow
VESSEL OCCLUSION
INDICATION
Hepatic artery and portal vein (Pringle maneuver)
Hepatic hemorrhage and surgery
Carotid artery
Nasal surgery, maxillectomy, lingual surgery; some brain procedures
Renal artery
Renal biopsy, nephrotomy
Femoral artery
Distal hemorrhage
Aorta
Cardiopulmonary resuscitation, cardiac surgery, setup of cardiopulmonary bypass, abdominal or thoracic hemorrhage
Tumor arterial supply
Tumor embolization/ local chemotherapy
Vena cava
Adrenalectomy with invasion of the vena cava
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Surgical Hemostasis
Only gold members can continue reading. Log In or Register to continue