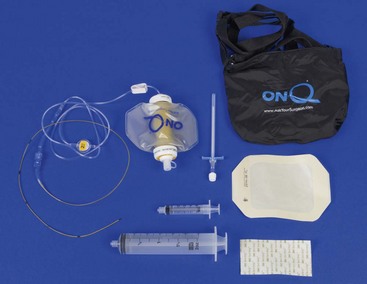
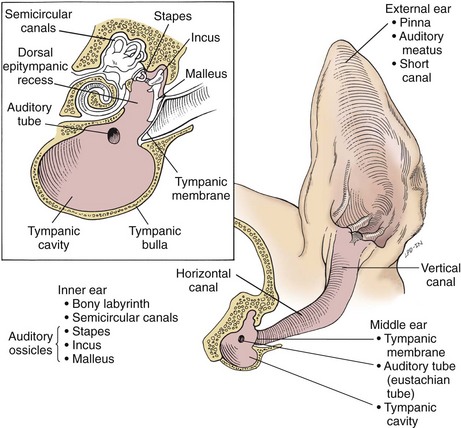
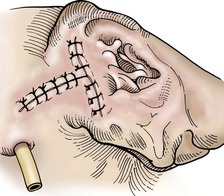
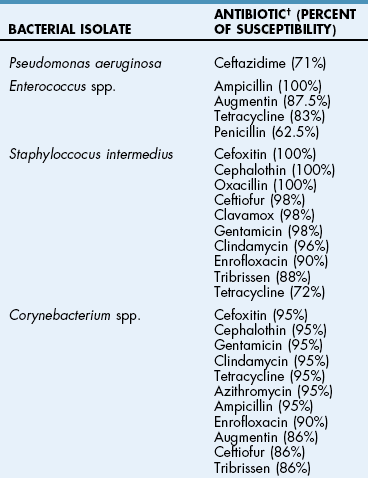
Chapter 18 General Principles and Techniques To anticipate surgical complications in animals undergoing ear surgery, it is imperative to determine the extent and severity of disease. Thickening and calcification of the ear canal indicate irreversible inflammatory disease. A sharp pain response on deep palpation of the ear may indicate middle ear infection, whereas a head tilt may indicate severe pain in the ear on the lower side or otitis media or interna (see p. 341). The latter conditions should be suspected if the head tilt is associated with circling, nystagmus, and/or vestibular dysfunction (loss of balance). Periorbital and retrobulbar abscesses may be associated with chronic otitis externa, media, and interna (Kraijer-Huver et al, 2009). Facial nerve deficits in patients with chronic otitis externa (i.e., poor palpebral reflex, lip droop, and facial spasms) suggest that the facial nerve is embedded in the horizontal canal, or that serious concurrent middle ear disease is present (see Chapter 38). Such abnormalities should be noted before surgery to avoid confusion with problems caused by intraoperative trauma during total ear canal ablation. Soft palate hypoplasia/malformation has been associated with middle ear disease in dogs (White et al, 2009). Otoscopic examination should determine whether the tympanic membrane is intact and should define the severity of change in the horizontal and vertical canals. Digital video-otoscopy under anesthesia is commonly used for diagnostic examination and treatment of the external ear canal (Rawlings, 2009). Always inspect both ear canals, even if the animal has unilateral clinical signs. Skull radiography or computed tomography (CT) should be performed to determine whether concurrent middle ear disease or neoplasia exists (see p. 343). Proliferation of cartilage or bone around the horizontal ear canal should be noted. After imaging, the ear should be cleaned; however, do not use chlorhexidine in a solution stronger than 0.2%, nondilute iodine or iodophors, ethanol, benzalkonium chloride, or some aminoglycosides if the tympanic membrane has ruptured. Drugs and solutions that may be used in animals with a ruptured tympanic membrane are listed in Box 18-1. Most animals undergoing ear surgery are healthy, and a variety of anesthetic protocols can be used. Ear surgery, particularly TECA, vertical canal resection, and lateral canal resection, is painful. Although butorphanol and buprenorphine are common premedicants in dogs, hydromorphone and morphine are better analgesics for dogs and cats undergoing ear surgery (Table 18-1). Saturating the surgical site with bupivacaine hydrochloride may provide some analgesia for 4 to 6 hours. A volume of bupivacaine sufficient to cover the area but not exceeding 2 mg/kg should be used. Contact time must be adequate (approximately 15 to 20 minutes) to allow the local anesthetic to work. The area needs to be relatively free of blood and should not be flushed during the 15 to 20 minutes that bupivacaine is in contact with the tissues. This technique should never be used as the sole method of pain control; it should always be used in conjunction with other analgesics. An alternative technique that provides prolonged postoperative analgesia is the constant-rate delivery catheter such as the ON-Q PainBuster (I-Flow Corp., Lake Forest, Calif.) local anesthetic system (Wolfe and Muir, 2003) (Box 18-2; Fig. 18-1) or the SUREFUSER (ReCathCo, Allison Park, Pa.), which is marketed for veterinary patients. The fenestrated catheter is placed in the open wound just before closure by extending the fenestrated end from one end of the wound to the opposite end. This catheter is then attached to an extension set, which consists of an air filter, a flow controller, extra tubing, and a clamp. The extension set is attached to the disposable bulb (the drug reservoir), which has a fill port. These bulbs and their corresponding flow controllers are designed to deliver a specific volume of local anesthetic per hour. Volume and flow rate are determined by identifying which bulb and flow controller are used. Bulbs come in a variety of sizes from 65 to 550 ml, and flow controllers can deliver rates of 0.5 to 10 ml/hr. Many ear surgery patients have chronic disease, allowing plenty of time to order and receive a system before surgery is performed. Doing so provides ongoing analgesia for several days while reducing narcotic requirements for a very painful procedure (see Box 18-2). Anesthetic Considerations in the Patient With Ear Disease *Monitor for hyperthermia in cats. †Buprenorphine is a better analgesic than morphine in cats. ‡Black box warning added by the FDA in October 2010 identified cases of renal failure and death in cats with repeated uses of meloxicam. Meloxicam is approved for single use only in cats in the United States. Although high doses of ketamine should not be given to patients with neurologic or renal dysfunction, a small ketamine bolus followed by a ketamine constant rate infusion (CRI) may facilitate intraoperative management and decrease postoperative pain in dogs or cats that have chronically painful ears (see Table 18-1). Alternatively, a single low-dose bolus of ketamine may be used to augment the pain protocol (see Table 18-1). In dogs, fentanyl, lidocaine, ketamine (FLK) or morphine, lidocaine, ketamine (MLK) CRI may be used intraoperatively or postoperatively for analgesia (see p. 138). Other useful CRIs in dogs include fentanyl and lidocaine; a fentanyl CRI without lidocaine may be used in cats. Postoperative analgesics should be given after ear surgery. If hydromorphone or morphine was used as a premedicant, it should be readministered 3 to 4 hours after the initial dose (see Table 18-1). If the animal appears dysphoric or anxious, tranquilization or benzodiazepines may be necessary; however, these medications should be used only in animals that have been given sufficient analgesics (see Table 18-1). If a question arises as to whether or not the animal is in pain, repeating analgesic therapy is nearly always warranted. Preoperative antibiotics are recommended in animals undergoing aural surgery. Severe infection should be treated with systemic and/or topical antibiotics for several weeks before surgery is performed, depending on the site of infection. Otitis externa is best treated with topical therapy because systemic antimicrobials are unlikely to achieve therapeutic concentrations within the fluid and exudates of the external ear canal. Commercially produced topical products typically contain one or more active ingredients (antibacterial, antifungal, or antiinflammatory) in various combinations plus a vehicle and various solubilizers, stabilizers, and surfactants. In contrast, systemic antibiotics are indicated in otitis media because the highly vascularized mucous membrane lining the tympanic cavity of the inflamed middle ear promotes diffusion of drugs from the blood to the bulla. The bulla may be packed with an antibiotic ointment and systemic antibiotics may be used if systemic antibiotics alone do not resolve the infection. Selection of systemic antibiotics for treating the middle ear compartment is preferably based on culture and susceptibility testing (Table 18-2). Cultures of deep tissues taken during surgery often are more useful than preoperative cultures. Percentage of Common Microbial Isolates Susceptible to a Tested Antimicrobial in Dogs With Chronic Otitis Externa* *From Graham-Mize CA, Rosser EJ: Comparison of microbial isolates and susceptibility patterns from the external ear canal of dogs with otitis externa, J Am Anim Hosp Assoc 40:102, 2004. †Only antibiotics with susceptibilities greater than 50% are listed. Initial treatment of otitis externa can be empirically based on historical information on the most common isolates and their susceptibility patterns in conjunction with examination of stained otic swab samples. When treatment failures occur, repeat examination and modification of treatment may be aided by repeat cytology of otic exudate and by submission of cultures. Malassezia spp. and Staphylococcus pseudintermedius are typically the most common microbial isolates identified in dogs with otitis externa. Most S. pseudintermedius organisms are susceptible to cephalothin and oxacillin. Most other bacterial isolates are susceptible to a number of antimicrobials; however, Pseudomonas aeruginosa and Enterococcus spp. are often fairly resistant bacteria (see Table 18-2). A recent study reported the use of a bacteriophage treatment in dogs with P. aeruginosa otitis (Hawkins et al, 2010). The authors noted that topical bacteriophage treatment led to bacterial lysis without apparent toxicity and suggested that this treatment had the potential to be convenient and effective for P. aeruginosa otitis in dogs. Otomycosis associated with Aspergillus spp. was recently reported in a dog that was unresponsive to topical and oral antibiotics and antifungal treatments (Coyner, 2010). Corynebacterium spp. have been reported as potential secondary pathogens in dogs with canine otitis externa (Aalbaek et al, 2010). If possible, ototoxic antibiotics (e.g., gentamicin, kanamycin, neomycin, streptomycin, tobramycin, amikacin, polymyxin B) should be avoided in animals with otitis. Tris-ethylenediaminetetraacetic acid (EDTA) solution or a combination of tromethamine, EDTA, and benzyl alcohol (see p. 340) may be beneficial in some animals with resistant infection. The ear is composed of three parts: (1) the inner ear, which consists of a membranous and bony labyrinth and functions for hearing and balance; (2) the middle ear, which is formed by the tympanic cavity and connects to the pharynx via the auditory tube (eustachian tube); and (3) the external ear, which is formed by the auditory meatus and a short canal (Fig. 18-2). The inner ear is located within the osseous labyrinth of the petrous part of the temporal bone. The middle and external ears are separated by the tympanic membrane, and the opening of the horizontal canal into the middle ear is known as the external acoustic meatus. The three auditory ossicles (stapes, malleus, and incus) connect the tympanic membrane to the inner ear. The tympanic cavity is air-filled and in dogs is composed of a small dorsal epitympanic recess and a large ventral tympanic bulla. In medium-sized dogs, the long axis of the tympanic cavity is about 15 mm. The auditory ossicles are found in the middle portion of the tympanic cavity. Vibrations of the tympanic membrane are transmitted through the chain of these auditory ossicles to perilymph fluid within the vestibule. The middle ear also connects to the nasopharynx via the auditory tube (commonly referred to as the eustachian tube). Nasopharyngeal polyps (see p. 353) may extend from the nasopharynx into the tympanic cavity and can extrude into the external ear canal. The feline tympanic cavity is divided into two compartments by a thin, bony septum that arises along the cranial aspect of the bulla and curves to attach to the midpoint of the lateral wall (Fig. 18-3). The larger ventromedial compartment is an air-filled tympanic bulla. For complete drainage of the middle ear of cats, this bony septum often needs to be perforated. Most of the lateral wall of the smaller craniolateral compartment is formed by the tympanic membrane. These compartments communicate through a narrow fissure located dorsally near the cochlear window. Near this fissure, the postganglionic sympathetic nerves form a plexus on a structure known as the promontory. Because of their vulnerable location, these nerves are often traumatized during surgical curettage of the feline middle ear, causing Horner’s syndrome (see p. 343). The tympanic membrane normally is thin and semitransparent but may thicken or rupture when diseased. The facial nerve exits the stylomastoid foramen caudal to the ear and courses ventral to the horizontal canal close to the middle ear. The external ear varies in size and shape among dog breeds. The auricular cartilage determines the appearance of the canine pinna. The base of the ear is composed of several ridges that are important landmarks for ear surgery (Fig. 18-4). These include the tragus, the lateral crus of the helix, the pretragic incisure, and the intertragic incisure. The external opening of the vertical canal is known as the external auditory meatus. Numerous muscles attach to the cartilage of the ear, allowing it to move to localize sound. The external ear canal consists of an initial vertical part and a shorter horizontal canal that runs medially. The vertical part and most of the horizontal part of the canal are cartilaginous; however, the deepest part (near the tympanic membrane) is osseous. The parotid salivary gland overlies the auricular cartilage, forming the vertical canal. Lateral ear canal resection increases drainage and improves ventilation of the ear canal. It also facilitates placement of topical agents into the horizontal canal. Lateral ear canal resection is indicated in patients with minimal hyperplasia of the ear canal epithelium or with small neoplastic lesions of the lateral aspect of the vertical canal. It should not be performed in animals with obstruction or stenosis of the horizontal ear canal or concurrent otitis media (unless performed in conjunction with ventral bulla osteotomy; see p. 335) or in patients with severe epithelial hyperplasia. Dogs with underlying disease (e.g., hypothyroidism, primary idiopathic seborrhea) often respond poorly to this surgery. Owner counseling is extremely important before a lateral ear canal resection is performed. Most studies have shown that owner satisfaction is low when lateral ear canal resection is performed for chronic otitis externa in dogs. A modification of the original technique for lateral ear resection, described by Lacroix, established a “drainboard” and is known as the Zepp procedure (Fig. 18-5). The drainboard restricts hair growth at the horizontal canal opening. Clip the entire side of the face and both sides of the pinna. Gently flush the ear and remove as much debris as possible. Position the animal in lateral recumbency with the head elevated on a towel and prepare the pinna and surrounding skin for aseptic surgery. Place quarter drapes around the ear with the entire pinna draped into the surgical site. Stand at the ventral aspect of the dog’s head and position a forceps into the vertical ear canal to determine its ventral extent. Mark a site below the horizontal ear canal that is half the length of the vertical ear canal (Fig. 18-6, A). Make two parallel incisions in the skin lateral to the vertical ear canal that extend from the tragus ventrally to the marked site (Fig. 18-6, B). These incisions should be Vertical canal ablation can be performed when the entire vertical canal is diseased but the horizontal canal is normal. It may be the technique of choice when neoplasia is confined to the vertical canal or in some animals with chronic otitis externa. Total removal of the vertical canal may result in less postoperative exudation and pain. This technique may provide a better cosmetic appearance of the ear than is provided by lateral ear canal resection when an abundance of hyperplastic tissue is present in and around the vertical canal (Fig. 18-7). Position and prepare the animal as for a lateral ear canal resection. Make a T-shaped incision with the horizontal component parallel and just below the upper edge of the tragus (Fig. 18-8, A). From the midpoint of the horizontal incision, make a vertical incision that extends to the level of the horizontal canal. Retract the skin flaps, reflect loose connective tissue, and expose the lateral aspect of the vertical canal (Fig. 18-8, B). Continue the horizontal incision through the cartilage around the external auditory meatus with a scalpel blade. Remove as much of the diseased tissue on the medial surface of the pinna as possible, but avoid damaging the major branches of the great auricular artery. Use curved Mayo scissors to dissect around the proximal and medial aspects of the vertical canal. During dissection, stay as close as possible to the cartilage of the ear canal to avoid inadvertently damaging the facial nerve. Free the entire vertical canal from all muscular and fascial attachments (Fig. 18-8, C). Transect the vertical canal ventrally 1 to 2 cm dorsal to the horizontal canal and submit it for histologic examination (Fig. 18-8, D). Incise the remnant of the vertical canal cranially and caudally to create dorsal and ventral flaps (Fig. 18-8, E). Reflect the ventral flap downward, and suture it to the skin for a drainboard using absorbable or nonabsorbable monofilament sutures (2-0 to 4-0). Suture the dorsal flap to the skin and close the subcutaneous tissue with an absorbable suture material (2-0 or 3-0). Then close the skin in a T shape (Fig. 18-8, F). TECA is indicated in animals with chronic otitis externa that has failed to respond to appropriate medical management, in cases of severe calcification and ossification of the ear cartilage, or when severe epithelial hyperplasia extends beyond the pinna or vertical ear canal (Fig. 18-9). The procedure commonly is performed on animals in which lateral ear resections have failed, and it may be beneficial for those with severely stenotic ear canals (Fig. 18-10). Neoplasia of the horizontal canal may be treated by TECA. In a study in cats, TECA was performed in 41% of cases because of neoplasia (typically ceruminous gland adenocarcinoma; see p. 350), whereas half of the cats had it performed because of chronic inflammatory or polypoid (see p. 353) disease (Bacon et al, 2003). Because of the potential for serious complications, this surgery should not be performed on animals with mild disease or by surgeons unfamiliar with the anatomy of the ear. A high percentage of dogs that undergo TECA have associated skin disease, such as seborrhea, atopy, or food or contact allergy dermatitis. The skin disease should be treated before surgery is planned because effective dermatologic therapy usually benefits the ears also. If the skin condition is unresponsive, TECA is preferred over lateral ear resection (see p. 330) and should be performed in conjunction with a lateral bulla osteotomy (see p. 335). A modified technique for performing TECA has been described; however, further investigation is warranted before the procedure can be recommended as a treatment for otitis externa not caused by masses or anatomic abnormalities of the horizontal ear canal in dogs with pendulous ears (Mathews et al, 2006). Pinna deformity after TECA in cats may be a source of dissatisfaction for some owners. A recently reported technique using a single-pedicle advancement flap at the base of the pinna during a modified TECA may facilitate upright ear carriage and improve the cosmetic result, and thus enhance owner satisfaction (Fig. 18-11). Position the animal in lateral recumbency with the head elevated with a towel. Prepare the pinna and surrounding skin for aseptic surgery. Make a T-shaped incision with the horizontal component parallel and just below the upper edge of the tragus (Fig. 18-12, A). From the midpoint of the horizontal incision, make a vertical incision that extends to just past the level of the horizontal canal (see Fig. 18-12, A). Retract the skin flaps, reflect loose connective tissue, and expose the lateral aspect of the vertical canal. Continue the horizontal incision around the opening of the vertical ear canal with a scalpel blade (Fig. 18-12, B). Use curved Mayo scissors to dissect around the proximal and medial aspects of the vertical canal (Fig. 18-12, C). During dissection, stay as close as possible to the cartilage of the ear canal to avoid inadvertently damaging the facial nerve. Avoid damaging the major branches of the great auricular artery at the medial aspect of the vertical canal. Identify the facial nerve as it courses caudoventrally to the horizontal canal (gently retract it if necessary). If the facial nerve is trapped within thickened, calcified horizontal canal tissue, carefully dissect the nerve from the horizontal canal. Continue the dissection to the level of the external acoustic meatus (Fig. 18-12, D). Excise the horizontal canal attachment to the external acoustic meatus with a scalpel blade, rongeur, or Mayo scissors, but be careful to avoid damaging the facial nerve. Remove the entire ear canal, and obtain deep cultures around or just inside the external acoustic meatus. Submit the ear for histologic examination. Use a curette to carefully remove secretory tissue that is adherent to the rim of the external acoustic meatus (Fig. 18-12, E). Be sure to remove all epithelial tissue in this region, or chronic fistulation will occur. Perform a lateral bulla osteotomy (see later discussion). Flush the area with sterile saline solution before closure. Close the subcutaneous tissue with absorbable suture (2-0 or 3-0), and close the skin in a T shape (Fig. 18-12, F). If drainage is desired, use blunt dissection to exit a Penrose drain ( Bluntly dissect the tissue from the lateral aspect of the bulla using a small periosteal elevator. Avoid damaging the external carotid artery and the maxillary vein, which travel just ventral to the bulla. Rongeur the lateral and ventral aspects of the bulla until the caudal aspect of the middle ear canal is exposed (Fig. 18-13). Extend the bony excision as needed to fully visualize the contents of the tympanic cavity, but avoid sharp dissection and curettage of the rostral aspect of the osseous ear canal to reduce the risk of retroauricular vein damage. Use a curette to remove infected material, but avoid curetting in the rostral (dorsal) or rostromedial area of the tympanic cavity so as not to damage the auditory ossicles or inner ear structures. Gently irrigate the cavity with saline to remove all remaining debris. Ventral bulla osteotomy allows increased exposure of the tympanic cavity and can be performed alone or in conjunction with lateral ear resection. It is the technique of choice when middle ear neoplasia is suspected in cats that have nasopharyngeal polyps (see p. 353). This technique provides better drainage of the bulla than does lateral bulla osteotomy and allows both bullae to be opened without the need to reposition the animal. Place the patient in dorsal recumbency, and prepare a generous area surrounding the angle of the mandible for aseptic surgery. Palpate the bulla immediately caudal and medial to the vertical ramus of the mandible. Draw an imaginary line connecting the mandibular rami and a second imaginary line along the long axis of the ventral aspect of the head (Fig. 18-14, A). In dogs, make a 7 to 10 cm incision (3 to 5 cm in cats) parallel to the midline of the animal and centered 2 cm toward the affected side from where these imaginary lines intersect (see Fig. 18-14, A). Incise the platysma muscle, retract the linguofacial vein if necessary, and deepen the incision by bluntly dissecting the digastricus muscle (lateral) from the hyoglossus and styloglossus muscles (medial). Avoid damaging the hypoglossal nerve, located on the lateral aspect of the hypoglossus muscle. Confirm the location of the bulla and use self-retaining retractors (e.g., Gelpi, Weitlaner) to spread the digastric and glossal muscles and retract them from the bulla (Fig. 18-14, B). Palpate the bulla craniomedial to the cornu process of the hyoid bone and caudomedial to the angle of the mandible. Bluntly dissect tissues from the ventral surface of the bulla and use a Steinmann pin to make a hole in its ventral aspect. Enlarge the opening with a small rongeur (e.g., Lempert). Examine the interior of the bulla for inflammatory debris, neoplastic tissue, or foreign bodies, and obtain samples for culture, sensitivity, and histopathologic examination. In cats, be sure to examine both compartments of the bulla (see the discussion on surgical anatomy, p. 329, and Fig. 18-3). Flush the cavity with warm saline; if evidence of infection is noted or, if continued drainage is anticipated, place a small, fenestrated drain tube in the cavity and exit it through a separate stab incision. Suture the fenestrated portion of the drain tube to the bulla with small chromic gut suture (4-0 to 6-0). Depending on the amount of exudation, remove the drain in 3 to 7 days. Postoperative analgesics should be given after ear canal resection or ablation (see the discussion on anesthetics on p. 325); tranquilizers may be administered if the animal appears dysphoric or anxious (see p. 326). A bandage should be placed over the ear or ears, and an Elizabethan collar or sidebar should be used to prevent bandage removal or ear mutilation. If swelling is excessive, a cold pack can be applied to the side of the face several times a day for the first 24 to 36 hours after surgery. Antibiotics should be based on culture results and continued for 3 to 4 weeks. Penrose drains generally can be removed in 3 to 7 days, and sutures can be removed in 10 to 14 days.
Surgery of the Ear
Preoperative Concerns
Anesthetic Considerations
![]() TABLE 18-1
TABLE 18-1

Antibiotics
![]() TABLE 18-2
TABLE 18-2
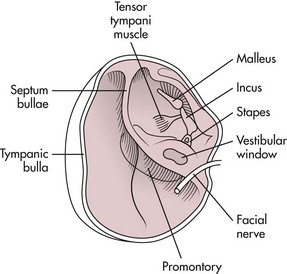
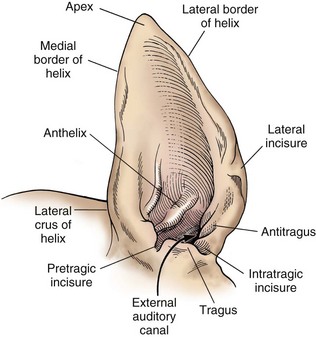
Surgical Anatomy
Surgical Techniques
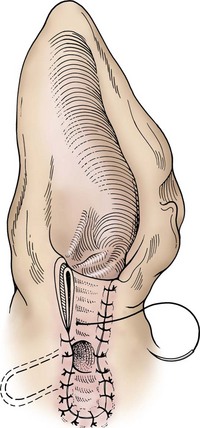
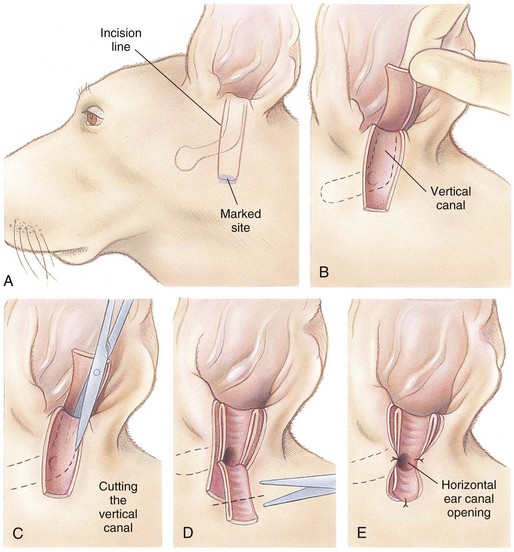
Lateral Ear Canal Resection
 times the length of the vertical ear canal. Connect the skin incisions ventrally and, using a combination of sharp and blunt dissection, reflect the skin flap dorsally, exposing the lateral cartilaginous wall of the vertical ear canal. During dissection, stay as close as possible to the cartilage of the ear canal to avoid inadvertently damaging the facial nerve. Note the parotid gland at the ventral extent of the incision and avoid damaging it. Standing at the dorsal aspect of the animal’s head, use Mayo scissors to cut the vertical canal (Fig. 18-6, C). Place one blade of the scissors in the canal at the pretragic or tragohelicine incisure at the cranial (or medial) aspect of the external auditory meatus and, with the scissors at a 30-degree angle, incise the canal ventrally to the level of the horizontal canal. Repeat the process beginning at the intertragic incisure (caudal or lateral aspect of the external auditory meatus). Do not allow the incisions to converge toward the lateral aspect of the canal, or the drainboard will be too narrow. Be sure to extend the incisions as far distally as the beginning of the horizontal canal, or the drainboard will not lie flat against the skin. Reflect the cartilage flap distally, and inspect the opening of the horizontal canal; if indicated, obtain cultures (Fig. 18-6, D). Occasionally, the opening can be widened by making two small cuts at the cranial and caudal aspects. Resect the distal half of the cartilage flap to make the drainboard and remove the skin flap. The ligament between the horizontal and vertical flaps usually acts as a hinge to allow the drainboard to lie flat, but in some cases scoring the cartilage on the ventral aspect of the drainboard facilitates this. Place absorbable or nonabsorbable monofilament sutures (3-0 or 4-0) from the epithelial tissue to the skin (Fig. 18-6, E). Begin suturing at the opening of the horizontal canal, then suture the drainboard. Last, suture the cranial and caudal aspects of the medial wall of the vertical ear canal to the skin (see Fig. 18-6, E).
times the length of the vertical ear canal. Connect the skin incisions ventrally and, using a combination of sharp and blunt dissection, reflect the skin flap dorsally, exposing the lateral cartilaginous wall of the vertical ear canal. During dissection, stay as close as possible to the cartilage of the ear canal to avoid inadvertently damaging the facial nerve. Note the parotid gland at the ventral extent of the incision and avoid damaging it. Standing at the dorsal aspect of the animal’s head, use Mayo scissors to cut the vertical canal (Fig. 18-6, C). Place one blade of the scissors in the canal at the pretragic or tragohelicine incisure at the cranial (or medial) aspect of the external auditory meatus and, with the scissors at a 30-degree angle, incise the canal ventrally to the level of the horizontal canal. Repeat the process beginning at the intertragic incisure (caudal or lateral aspect of the external auditory meatus). Do not allow the incisions to converge toward the lateral aspect of the canal, or the drainboard will be too narrow. Be sure to extend the incisions as far distally as the beginning of the horizontal canal, or the drainboard will not lie flat against the skin. Reflect the cartilage flap distally, and inspect the opening of the horizontal canal; if indicated, obtain cultures (Fig. 18-6, D). Occasionally, the opening can be widened by making two small cuts at the cranial and caudal aspects. Resect the distal half of the cartilage flap to make the drainboard and remove the skin flap. The ligament between the horizontal and vertical flaps usually acts as a hinge to allow the drainboard to lie flat, but in some cases scoring the cartilage on the ventral aspect of the drainboard facilitates this. Place absorbable or nonabsorbable monofilament sutures (3-0 or 4-0) from the epithelial tissue to the skin (Fig. 18-6, E). Begin suturing at the opening of the horizontal canal, then suture the drainboard. Last, suture the cranial and caudal aspects of the medial wall of the vertical ear canal to the skin (see Fig. 18-6, E).
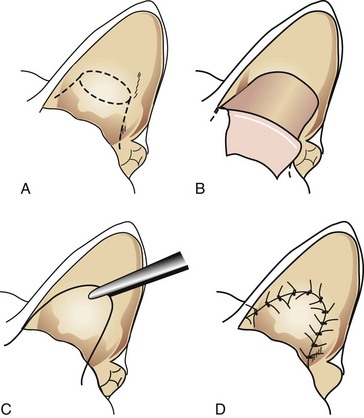
Vertical Ear Canal Ablation
![]() Total Ear Canal Ablation
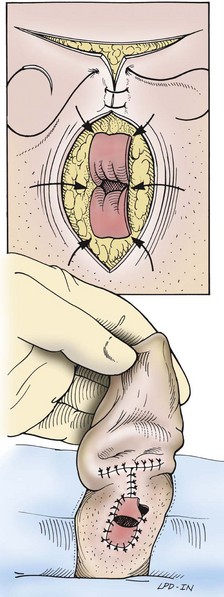
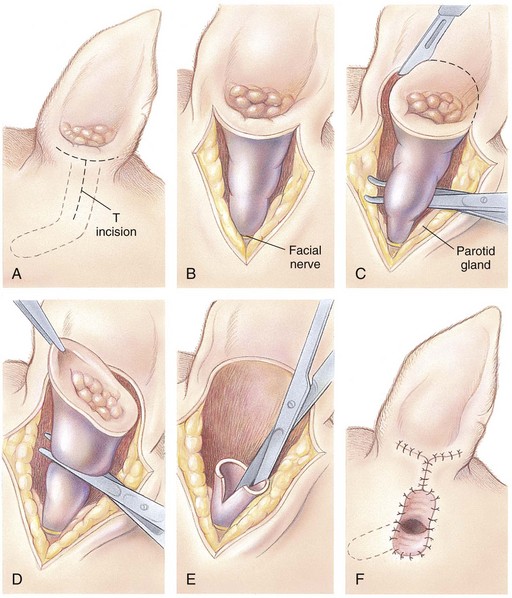
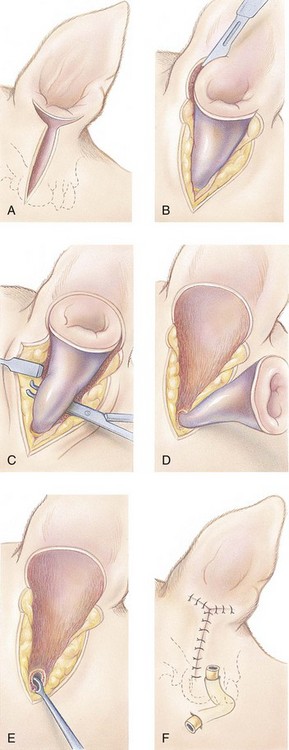
Total Ear Canal Ablation
 to
to  inch wide) or soft rubber tubing ventral to the incision in a dependent area (through a separate stab incision), or use closed suction drainage (e.g., butterfly catheter, Vacutainer tube). The end of the drain near the tympanic cavity may be secured with a single suture of chromic catgut (4-0 or 5-0). Secure the drain to the skin at the exit site.
inch wide) or soft rubber tubing ventral to the incision in a dependent area (through a separate stab incision), or use closed suction drainage (e.g., butterfly catheter, Vacutainer tube). The end of the drain near the tympanic cavity may be secured with a single suture of chromic catgut (4-0 or 5-0). Secure the drain to the skin at the exit site.
Lateral Bulla Osteotomy
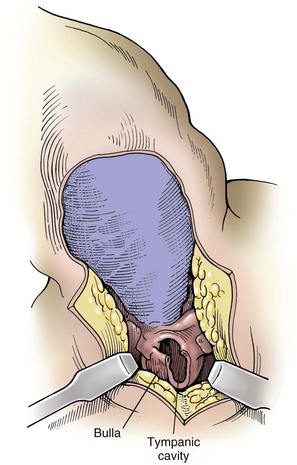
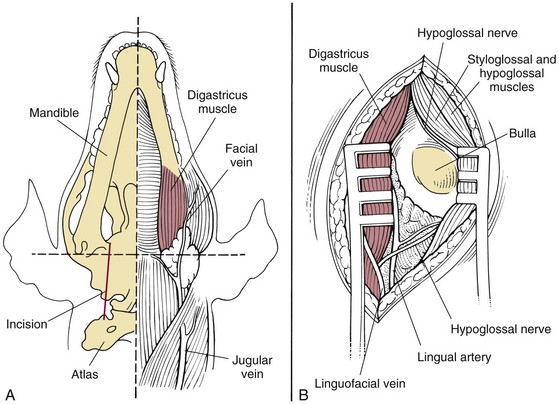
Ventral Bulla Osteotomy
Postoperative Care and Assessment
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Surgery of the Ear