Chapter 8 Surgery of the cornea and sclera
Preoperative treatment considerations 194
Surgical instrumentation for corneal and scleral surgeries 195
Surgical procedures for superficial corneal diseases 196
Surgical procedures for deep corneal ulcerations 201
Surgery for corneal lacerations 210
Surgery for corneal foreign bodies 214
Corneal grafts/keratoplasty 216
Surgical treatment of limbal and scleral diseases 229
Surgical anatomy
Dog and cat corneal anatomy
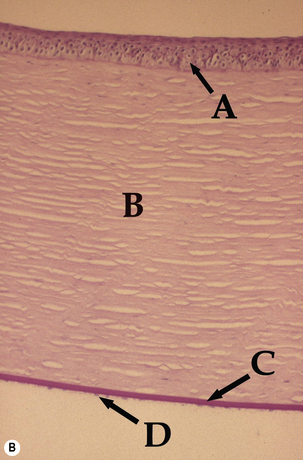
The cornea, along with the sclera, forms the fibrous tunic of the globe (Fig. 8.1). The zone where the cornea gradually becomes opaque and changes to sclera is the limbus. The limbus is sufficiently forward of the aqueous humor filtration angle or the iridociliary cleft (or ciliary cleft) to prevent direct visualization of the aqueous outflow pathways. Dog and cat corneas are divided into axial (central) and peripheral, with the central area most important for vision. Often the cornea is divided into quadrants. The central cornea is generally the thinnest and most often affected with ulcerations.
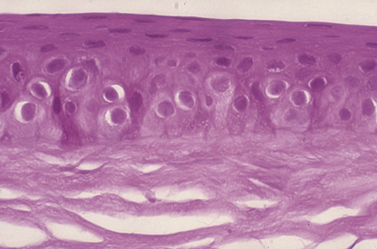
Dog and cat corneas have four different microscopic regions. From external to internal, these subdivisions include: 1) epithelia with basal membrane; 2) thick stromal layer; 3) Descemet’s membrane; and 4) the posterior single layer endothelia. A modified anterior region of the corneal stroma, Bowman’s membrane, is absent in the dog and cat, but present in humans and most birds. The epithelial layer is normally about 5–7 cells thick and consists of: 1) outer two to three layers of non-keratinized squamous cells; 2) middle two to three layers of polyhedral or wing cells; and 3) a single layer of basal columnar cells that are positioned on a basement membrane (Fig. 8.2). The apparent turnover of corneal basal epithelia is about 7 days. The basement membrane, produced by the basal corneal epithelia, attaches the basal epithelial cells via hemidesmosomes to the anterior stroma. Defects in the canine basal corneal epithelia and basement membrane are thought to contribute directly to the development of recurrent corneal erosions. The corneal stroma, or substantia propria, accounts for about 90% of the corneal thickness, and consists of parallel bundles of collagen fibrils, few fibrocytes (also called keratocytes), and a matrix of glycosaminoglycans. The arrangement of these fibrils and the matrix of glycosaminoglycans become distorted with disease and is the basis of corneal opacification.
Preoperative treatment considerations
Dog and cat
The dog and cat eyes rotate during gas anesthesia ventromedially, and collapse once the anterior chamber has been opened. The use of non-depolarizing neuromuscular blocking agents such as atracurium (0.2 mg/kg IV; Glaxo-Wellcome Research, Triangle Park, NC) paralyzes the animal, including the extraocular muscles. As a result, the eye position remains normal and the lack of extraocular muscle tone reduces intraocular tissue displacement once the anterior chamber is entered. Because of the paralysis of all striated musculature, including the muscles associated with breathing, artificial ventilation in these anesthetized patients is essential until the drug-induced paralysis ceases or is reversed (see Chapter 3).
Surgical instrumentation for corneal and scleral surgeries
Small animals
The investment in surgical instruments depends on the expertise of the veterinary surgeon and the anticipated patient load with corneal surgical diseases (see Table 1.4, p. 12). Magnification is essential for corneal surgeries. Head loupes can provide magnification at levels of 2.5 to 4×; the operating microscope provides at least 10× and is generally preferred. With considerable corneal surgery and keratoplasty, the operating microscope is recommended. Either standard or microsurgical instruments may be used, or a combination of both sizes. Minimal instruments include the following.
• For exposure of the cornea and a possible lateral canthotomy: tenotomy or Steven’s scissors, eyelid speculum, tissue forceps (Bishop–Harmon), and needle holder (standard; with lock: Castroviejo). For additional information on lateral canthotomy, see Chapter 2.
• For corneal tissues: Colibri and tying forceps (with 1 × 2 teeth), Beaver scalpel handle and blades (Nos 6400, 6500, and keratome), corneal scissors (right and left handed), iris scissors, disposable electrocautery, Martinez corneal dissector, associated cannulas, needle holder (microsurgical size and without lock), and a set of corneal trephines (at 0.5 mm increments). The diamond knife is very useful for corneal surgery and can provide exact control of the depth of the corneal incision in increments of 0.2, 0.3, 0.4, 0.5, 0.6 and 0.8 mm.
• For keratoplasty, additional instruments include corneal cutting block (often Teflon®) and punch. At least one or two different sizes of Flieringa rings are useful for corneal transplantation, and are temporarily sutured to the bulbar conjunctiva and episclera to prevent collapse of the globe (see Chapter 1).
Surgical procedures for superficial corneal diseases
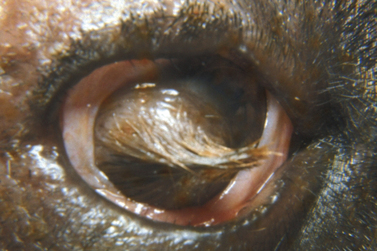
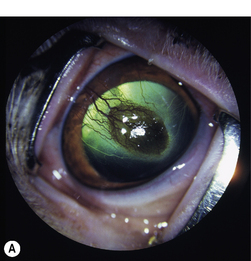
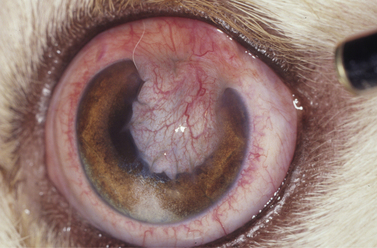
Superficial corneal diseases are usually confined to corneal epithelia and anterior stroma, and may be treated surgically. For instance, corneal dermoids generally extend into the anterior stroma and may involve adjacent bulbar conjunctiva (Fig. 8.3). Treatment is superficial keratectomy. Corneal lipidosis often affects the anterior corneal stroma, and may be removed by superficial keratectomy. Recurrent corneal erosions in the dog appear related to corneal epithelial dystrophy and defects in the basement membrane which result in defective adhesion during healing of these superficial erosions and frequent recurrences (Fig. 8.4). Several surgical procedures, including superficial keratectomy, have been used to treat this condition. The superficial keratectomy procedure may be used for corneal sequestra in cats limited to the anterior corneal stroma.
Superficial keratectomy (partial and complete)
Indications for superficial keratectomies include corneal dermoid, chronic superficial keratitis (pannus), pigmentary keratitis, chronic and recurrent corneal erosions, corneal and/or limbal neoplasia, ulcerative keratitis with sequestration in cats (Fig. 8.5), corneal superficial dystrophies (usually lipidosis and calcification), and superficial corneal foreign bodies. In some of these diseases, the cause(s) has not been determined, and although the involved corneal tissue(s) appears completely excised, recurrence may occur. For some of these conditions, such as pigmentary keratitis, the superficial keratectomy may re-establish a clear cornea, but if the predisposing factors, such as lagophthalmia, nasal fold trichiasis, or tear film disorder, are not resolved, the cornea will become pigmented again.
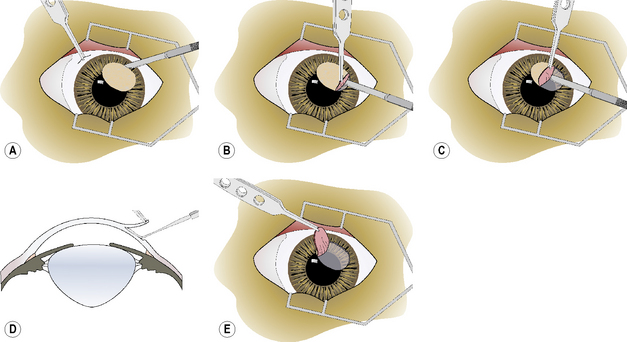
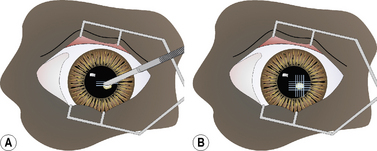
Superficial keratectomy is usually limited (partial) to the diseased cornea. The periphery of the diseased area is encircled with an incision using the Beaver scalpel handle and No. 6400 microsurgical blade, or the diamond knife with the micrometer set at 0.15 or 0.25 mm, or a preset corneal trephine (0.15–0.25 mm). The incision should be of sufficient depth to remove the base of the diseased cornea, as estimated preoperatively by slit-lamp biomicroscopy (Fig. 8.6a). Often, the cornea is vascularized, and limited hemorrhage occurs during the incision. To prevent hemorrhage from obscuring the incision, a continuous stream of 0.9% sterile saline is directed at the leading aspects of the corneal incision as it is performed. This hemorrhage will usually cease once sufficient time has elapsed to permit clotting. If a conjunctival graft is applied to the keratectomy site, the surgical defect may be constructed as a square lesion.
Once the corneal lesion has been outlined, the edge of the superficial keratectomy section is grasped carefully with 1 × 2 teeth tissue forceps to permit separation of the diseased cornea from the underlying stroma (Fig. 8.6b). The dissection plane within the stroma should remain in the same parallel lamellae throughout the superficial keratectomy. If the Beaver knife is used, the instrument must be held tangential to the corneal stroma to prevent progressive deeper dissection into the stroma (Fig. 8.6c). Alternately, the Martinez dissector facilitates this dissection to remain within the respective corneal lamellae (Fig. 8.6d).
Once the diseased cornea has been completely separated from the stroma, it is lifted from the surgical wound (Fig. 8.6e). If some tags of stroma remain, they are carefully cut with utility or tenotomy scissors. If additional areas of diseased cornea are still present, the procedure may be repeated in these areas.
Adaptations in large animals and special species
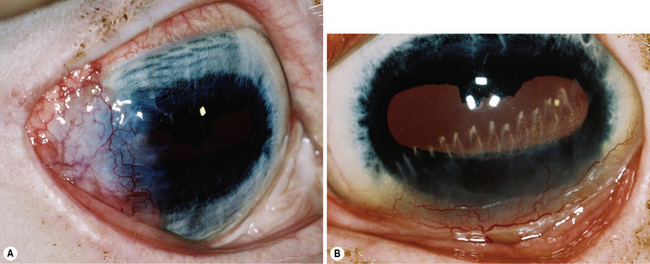
Superficial keratectomy is also performed during any grafting procedure to prepare the bed for conjunctival, amniotic membrane or corneal graft. The complications of superficial keratectomy are minimal, but include slow healing, infection, granulation tissue formation, perforation, and excessive scar formation. Superficial keratectomy may also be used in the treatment of superficial corneal scarring, with postoperative treatment of the corneal healing response (Fig. 8.7).
Superficial punctate, grid, and linear keratotomies
Superficial punctate, grid, and linear keratotomies are relatively new surgical procedures used to treat chronic corneal erosions and refractory corneal ulcers in dogs and other species (Fig. 8.8). Investigations into corneal recurrent erosions (indolent ulcer or Boxer ulcer) in the dog indicate defective basal corneal epithelia and basement membrane. Defects in the basement membrane, including paucity of hemidesmosomes and multiple layers of basement membrane, appear to contribute directly to the onset of these highly painful but shallow corneal ulcers, and to their variable but often prolonged healing. Both surgical procedures attempt to improve adhesion of the defective epithelia and basement membrane to the anterior stroma by making multiple shallow grooves in the epithelia and anterior stroma that provide deeper attachment sites. As a result, basal corneal epithelia increase their surface contact and adhesion through these incomplete needle or linear tracks to the anterior stroma. As the entire cornea is involved, these procedures are used for most of the entire cornea including the actual erosion site. Punctate keratotomy leaves smaller scars than grid keratotomy. The keratotomy procedures are usually preceded by debridement using a cotton swab and topical anesthesia of the edges of these chronic erosions for 1 or 2 weeks. If epithelialization has not occurred in 12–14 days, one of these keratotomies is performed. Partial-to-complete superficial keratectomy has also been used for this condition. Other surgical procedures for recurrent and slow healing corneal erosions, such as transplantation of small lenticules of new and healthy epithelium (keratoepithelioplasty), microdiathermy of Bowman’s membrane, and neodymium:yttrium aluminum garnet (Nd:YAG) laser photo-induced adhesion of corneal epithelia, have not been reported in the dog.
Superficial punctate keratotomy
In superficial punctate keratotomy, multiple anterior stromal punctures are performed with a 20–23 g disposable hypodermic needle or a diamond corneal knife with the micrometer set at 0.10–0.2 mm (Fig. 8.9a). The hypodermic needle is grasped directly or clamped with a small hemostat to expose 0.1–0.2 mm of the tip. The needle should enter the cornea at a 45–90° angle. The 23 g hypodermic needle will penetrate deeper into the corneal stroma than the 20 g hypodermic needle. Alternatively, the Nd:YAG laser may be used with multiple impulses set at 2 mJ.
About 15–25 punctures are usually made about 1.5 mm apart surrounding the erosion and extending into the adjacent normal cornea (Fig. 8.9b). The cornea is slightly indented when the 0.1–0.2 mm depth is achieved. If inadvertent complete puncture of the cornea results, rapid self-sealing occurs.
Superficial grid keratotomy
In the superficial grid keratotomy procedure, the corneal epithelia and anterior stroma are incised numerous times in a grid, cross-hatching or linear pattern. The majority of the grid incisions are adjacent to the corneal erosion, but this procedure may cover most of the corneal surface. The linear incisions for superficial grid keratotomy are made with a 20 g disposable hypodermic needle, Beaver No. 6400 microsurgical blade, or a diamond knife with the micrometer set at 0.2–0.3 mm deep (Fig. 8.10a). Incisions at 90° to the first series of incisions complete the grid keratotomy (Fig. 8.10b). Smaller gauge hypodermic needles are not recommended, as their incisions extend too deep. The grids are about 1–1.5 mm apart.
Superficial linear keratotomy
Alternative treatments appear to yield lower success rates. Treatment of canine recurrent erosions with only aqueous iodine cautery of the erosion requires an average of 46 days for complete re-epithelialization. Contact lenses for corneal erosions yield 73% success, the major limitation being retention of the lens. If the lens is retained for 7–10 days, the success rate increases to 92%. Other forms of treatment for this disorder include nictitating membrane flaps, temporary tarsorrhaphy, and bulbar conjunctival grafts (see Chapters 5 and 6). The success rates of these treatment methods have focused on short-term management of the healing of the recurrent erosions. The real value of these procedures, yet to be established, is long-term prevention of recurrent corneal erosions.
Corneal biopsy
Adaptation for large animals and special species
Stromal biopsies to aid in the diagnosis of stromal abscesses or immune-mediated keratopathies in horses can be obtained in the standing horse. Utilizing sedation and topical anesthesia, a linear incision in the corneal epithelium is made with the Beaver No. 6400 or 6900 microsurgical blade (Fig. 8.11). A Martinez corneal dissector is then used to separate the epithelium from the stroma. A small biopsy punch is utilized to obtain a stromal sample, and the tissue placed on a sponge in a histologic cassette in fixative. The corneal epithelium is sutured with 8-0 sutures in a simple interrupted pattern. Topical antibiotics and NSAIDs can be used post-biopsy.
Surgical procedures for deep corneal ulcerations
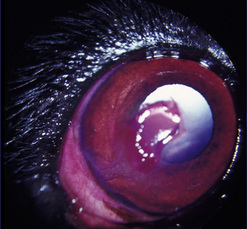
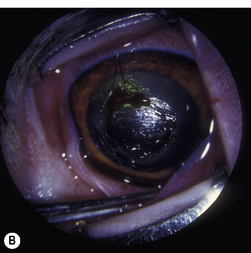
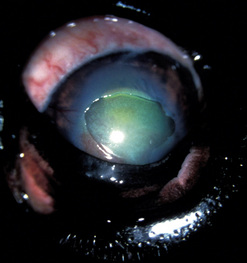
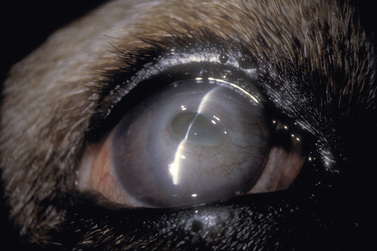
Corneal ulcerations are frequent in dogs but less common in cats. In the dog, corneal ulcers may be initiated by minor trauma. In certain breeds, particularly brachycephalic dogs, corneal ulceration may be associated with several predisposing factors. In brachycephalic breeds the eye is very prominent, suffers lagophthalmia, and the rate of blinking may be less than normal. Corneal microtrauma may occur from distichia and nasal fold trichiasis. The precorneal film may be thin and abnormal centrally, placing the central corneal epithelia in constant jeopardy. Retention of rose Bengal by the central corneal epithelia in these dogs suggests that these cells are degenerating at a faster rate than normal. These eyes are frequently also victims of marginal or low levels of aqueous tear production. The combinative effect is the development of a central-to-paracentral corneal ulcer that rapidly becomes deeper and larger (Fig. 8.12).
Primary closure of small deep corneal ulcers
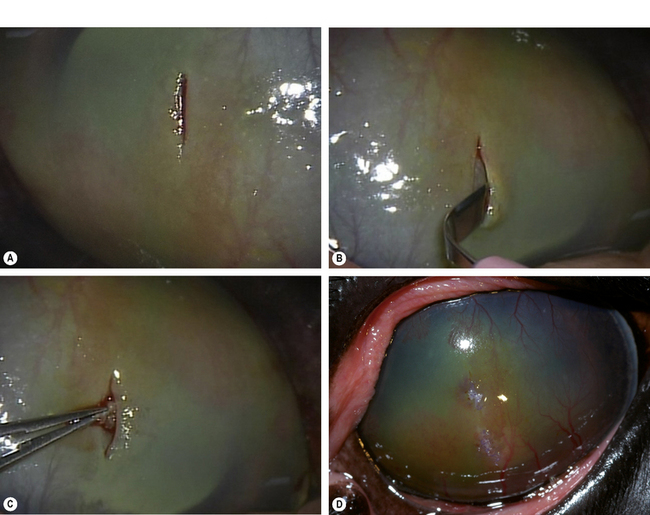
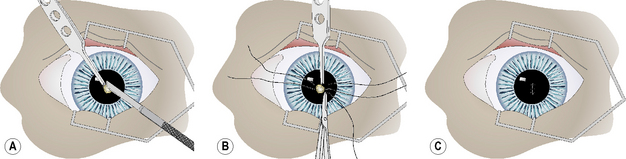
Small deep corneal ulcers may be closed by direct suturing. The maximum diameter of corneal ulcers that can be apposed by sutures is about 3 mm or less. Control and hopefully elimination of the potential pathogenic bacteria from the ulcer site contributes directly to the success or failure of this method. After general anesthesia and surgical preparation of the eyelids and conjunctiva, the eye is draped and an eyelid speculum positioned. The corneal ulcer is closely examined, and any necrotic or suspect tissue carefully removed by Beaver No. 6400 microsurgical blade (Fig. 8.13a). These tissues should be cultured and evaluated histologically. The edges of the corneal ulcer are carefully treated with aqueous 0.5% povidone–iodine solution applied with sterile cotton swabs to attempt sterilization of the ulcer. The same solution may be applied carefully to the ulcer base, but only if some deep corneal stroma remains.
Two or three 5-0 to 6-0 braided polyglactin 910 simple interrupted sutures, or a combination of a central interrupted horizontal mattress suture and two simple interrupted sutures, are used to appose the ulcer’s edges (Fig. 8.13b). Sutures are placed into the deep corneal stroma. Some distortion of the cornea develops as the sutures are tied and ulcer edges apposed (Fig. 8.13c). Fortunately, as corneal healing occurs during the next 7–10 days, the corneal curvature gradually returns to normal.
Conjunctival autografts
Conjunctival autografts are frequently used in small animal ophthalmology for the clinical management of deep and large corneal ulcers, descemetoceles, mycotic ulcers, stromal abscesses, after sequestrum removal in cats, keratomalacia, and for perforated corneal ulcers with and without iris prolapse. Conjunctival autografts were presented in Chapter 7. Conjunctival autografts consist of either bulbar or palpebral conjunctival mucosa with epithelium, and connective tissue. These autografts can be transposed and sutured directly to the edges of the corneal ulcer or defect to provide additional support and tissue for a cornea weakened by deep ulceration, descemetocele, or perforation with or without iris prolapse. The transplanted conjunctival autograft provides additional tissues and no risk of host rejection.
Conjunctival autografts from either bulbar or palpebral conjunctiva should be thin, and not include Tenon’s capsule or the bulbar fascia. The inclusion of Tenon’s capsule may contribute to surgical failure by increasing the traction on the transplanted conjunctival graft. Transpalpebral conjunctival autografts contain limited portions of the fibrous tarsal layer which may be necessary to maintain the graft base from the deeper aspects of the eyelid to the corneal surface. Conjunctival autografts are more difficult to perform than nictitating membrane flaps, but are easier than corneoconjunctival and corneoscleral transpositions, and the different types of keratoplasty. The most frequent type of bulbar conjunctival graft is the pedicle type (Fig. 8.14).
Nictitating membrane flaps
Nictitating membrane flaps provide more support to the diseased cornea than temporary complete tarsorrhaphy in small animals. Nictitating membrane flaps are used to cover and protect a weakened cornea, but are not usually a source of tissues for the cornea. Nictitating flaps are recommended for superficial corneal diseases, including corneal erosions, neuroparalytic and neurotropic keratitis, temporary exposure keratitis, superficial corneal ulcers, and acute keratoconjunctivitis sicca, and to reinforce a bulbar conjunctival graft. Surgical procedures for nictitating membrane flaps are presented in Chapter 7.
Corneoscleral transposition
Corneoscleral transposition shifts the peripheral cornea into central corneal defects and moves adjacent sclera into the peripheral cornea. As this procedure is primarily used for the repair of corneal ulcerations it is included in this section; however, corneoscleral and corneoconjunctival transpositions may also be considered as distinct types of autologous sliding lamellar keratoplasty. As a result, axial (central) and visually important cornea remains clear, but the peripheral cornea with the transposed sclera becomes somewhat translucent. A modification of this surgery, the corneoconjunctival autograft, was presented in Chapter 7. Corneoscleral transpositions are used to treat deep corneal ulcerations, descemetoceles, and feline corneal sequestra involving the deeper stroma. The advancement of corneoscleral tissues into central corneal ulcers, suspected as septic or not treated with antibiotics, is not recommended as these grafts may also be destroyed with the progressive melting process. If the tip of the corneoscleral graft is already vascularized, the chance of slough from infectious agents is reduced. Hence, before corneoscleral transpositions are performed, the corneal ulcer’s edges should be scraped and examined microscopically for bacterial and fungal organisms. Alternatively, the corneal ulcer may be treated hourly with topical broad-spectrum antibiotics for several hours to attempt sterilization of the ulcer site.
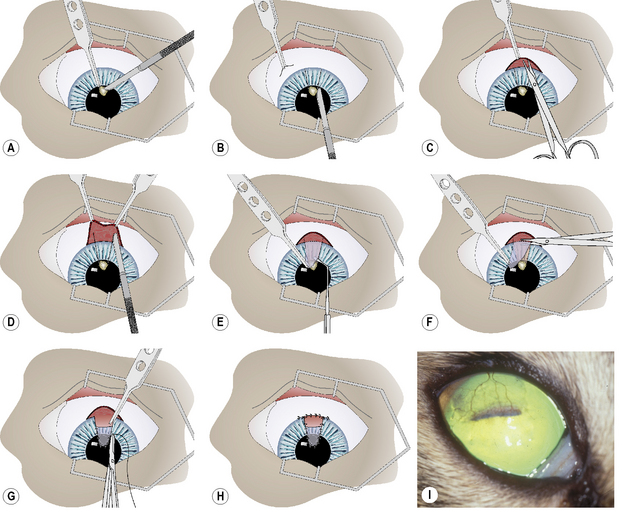
After the onset of general anesthesia, and surgical preparation of the eyelids, conjunctiva, and cornea with aqueous 0.5% povidone–iodine solution, the eye is draped and an eyelid speculum positioned. The corneal ulcer is carefully debrided to remove all potentially necrotic and/or infected tissues (Fig. 8.15a). Once these tissues have been removed, the corneal defect may be 1–2 mm larger. The corneoscleral advancement graft is prepared. Two slightly diverging corneal incisions with the Beaver No. 6400 microsurgical blade are performed, extending from the corneal bed to the limbus (Fig. 8.15b). These incisions are approximately one-half of the stromal thickness. At the limbus, the bulbar conjunctiva and Tenon’s capsule are incised by tenotomy scissors for about 15–20 mm and reflected caudally to expose the sclera (Fig. 8.15c).
The ends of the two corneal incisions are extended into the sclera at a distance equal to the height of the corneal ulcer bed (Fig. 8.15d). These incisions should be about 0.2–0.3 mm deep. Hemorrhage during these incisions is anticipated and judicious cautery with the disposable battery-powered electrocautery unit is necessary for hemostasis. The tip of the corneoscleral transposition is elevated with 1 × 2 teeth thumb forceps and a corneal dissector, and separated from the corneal bed to the end of the scleral incisions (Fig. 8.15e). The corneal separator, in contrast to sharp dissection with a scalpel blade, facilitates dissection within the corneal lamellae without the danger of shifting the plane of tissue separation.
Once separation from the underlying corneosclera is complete, the base of the graft is incised with tenotomy scissors (Fig. 8.15f). The length and width of the tip of the corneoscleral graft should be 1–2 mm larger than the corneal ulcer bed. The corneoscleral graft is positioned in the corneal ulcer bed, trimmed if necessary, and apposed with 7-0 to 9-0 simple interrupted braided polyglactin 910 sutures (Fig. 8.15g). The edge of the bulbar conjunctiva is apposed back to the limbus with a 7-0 to 9-0 simple continuous braided polyglactin 910 suture (Fig. 8.15h).
Postoperative treatment after corneoscleral transpositions includes topical and systemic broad-spectrum antibiotics, and topical mydriatics sufficient to maintain a moderately dilated and mobile pupil. After 7–10 days, topical corticosteroids are started to reduce corneal scarring. Clearing of the cornea usually starts 2–6 weeks postoperatively. Success rates with corneoscleral transpositions are 75– 80% (Fig. 8.15i).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree