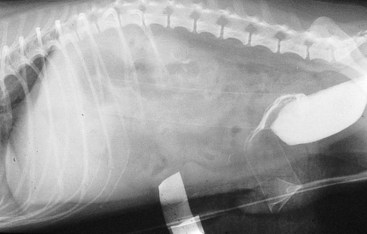
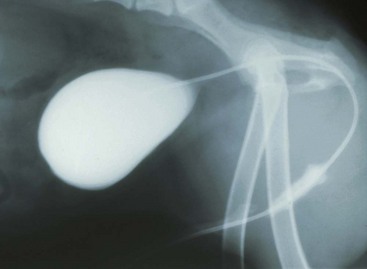
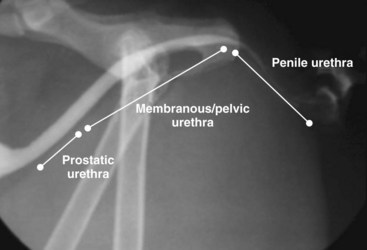
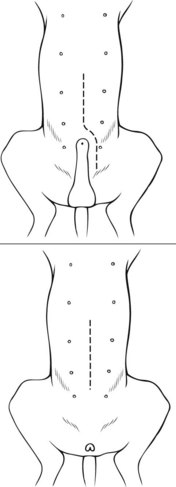
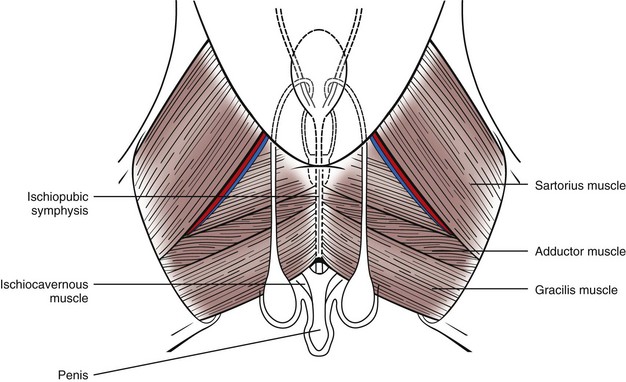
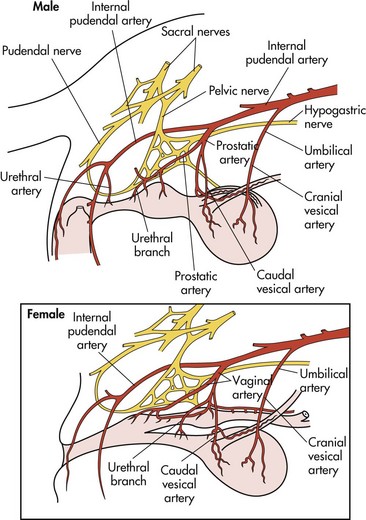
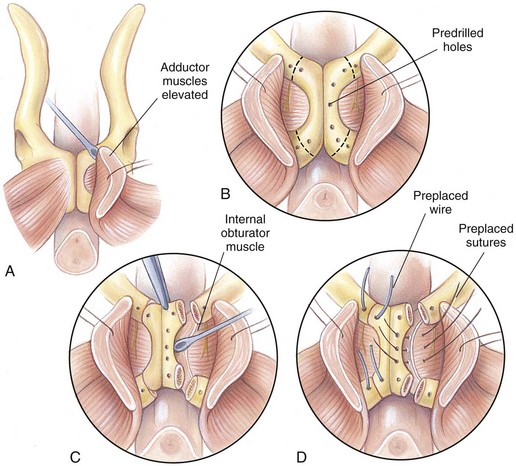
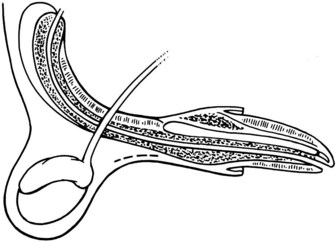
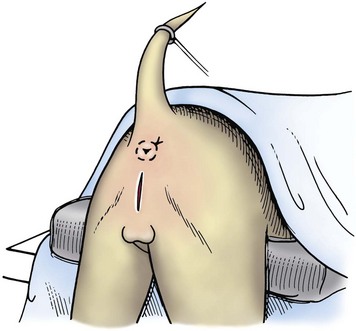
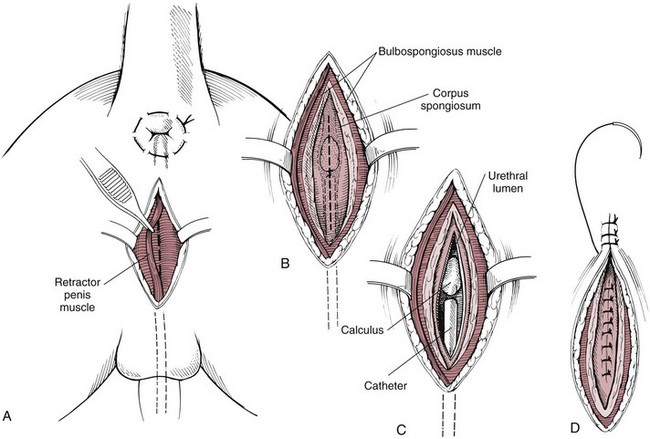
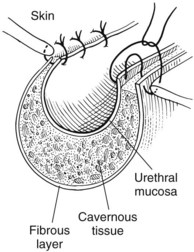
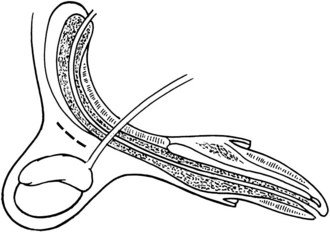
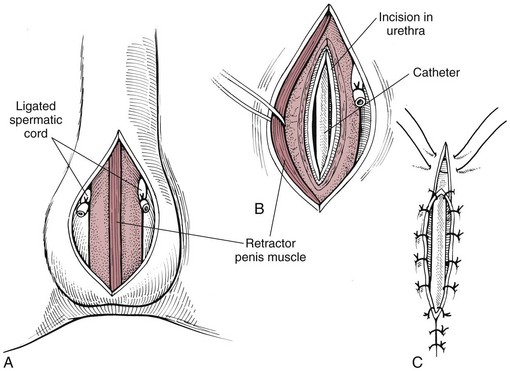
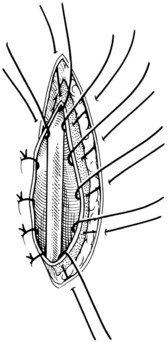
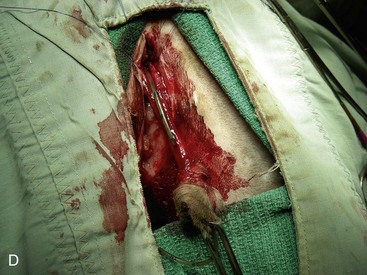
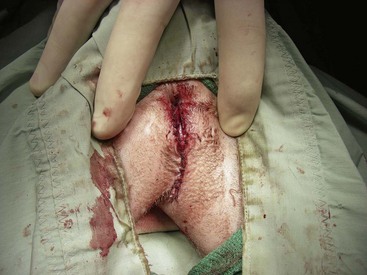
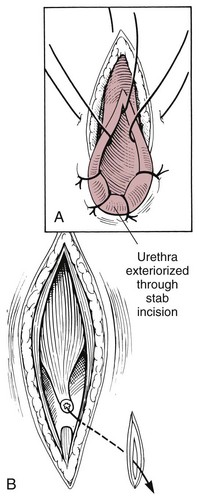
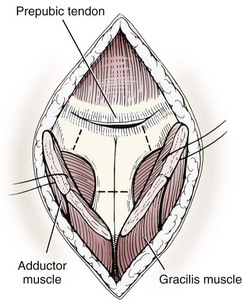
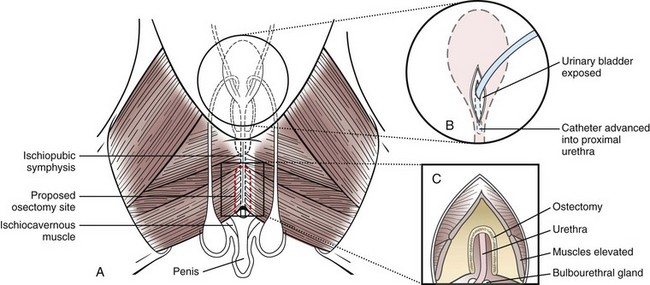
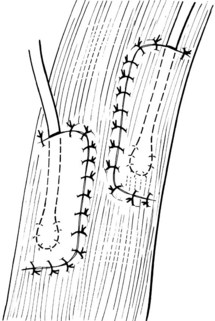
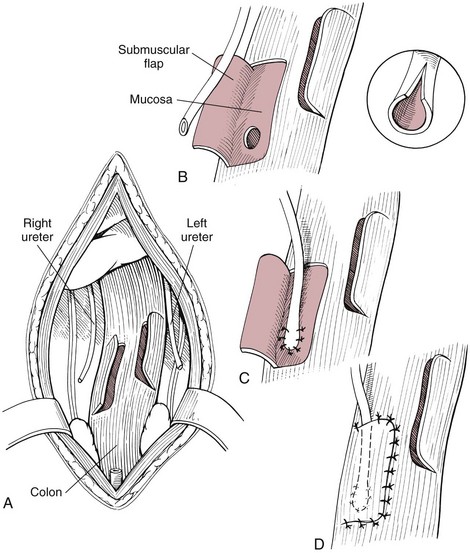
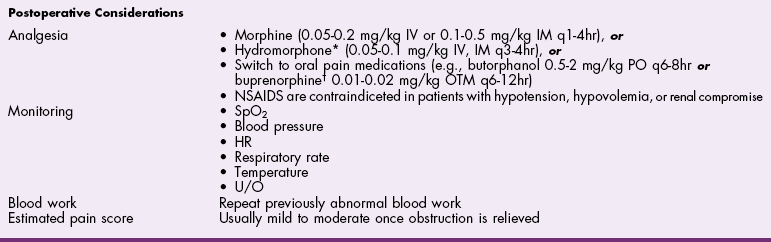
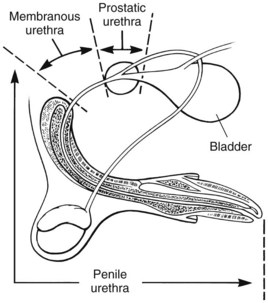
Chapter 26 General Principles and Techniques Cystolithiasis, neoplasia, and rupture are the most common abnormalities of the urinary bladder in small animals. Urinary obstruction may occur if calculi become lodged in the urethra, or if a tumor obstructs the proximal urethra or trigone. Male cats with FIC may develop penile urethral obstruction (see p. 777). Obstruction to urinary flow may cause a distended urinary bladder, postrenal azotemia, and hyperkalemia. Bladder rupture primarily occurs after motor vehicular trauma but may also be caused by necrotic bladder (e.g., following damage to its blood supply or prolonged urethral obstruction) or as a complication of bladder surgery (Fig. 26-1). Urinary leakage into the abdominal cavity causes uremia, dehydration, hypovolemia, hyperkalemia, and death if undiagnosed or untreated. Urinary obstruction and uroperitoneum are medical emergencies, not surgical emergencies. Hyperkalemia associated with these conditions makes the animal prone to cardiac arrhythmias; therefore fluid and electrolyte abnormalities should be corrected before anesthesia (see p. 736). Hyperkalemia causes bradycardia, absent or flattened P waves, prolongation of the P-R interval, widened QRS complexes, and/or “tented” or spiked T waves, in addition to predisposing to cardiac arrhythmias. Potassium concentrations greater than 7 mEq/L may cause irregular idioventricular rhythms, and potassium concentrations exceeding 9 mEq/L commonly cause atrial standstill. Mild or moderate hyperkalemia may be treated with intravenous (IV) fluids (i.e., 0.9% saline for dilution; Box 26-1). Both lactated Ringer’s solution (LRS) and 0.9% saline have been shown to be effective in resolving metabolic acidosis, hyperkalemia, and postrenal azotemia associated with urethral obstruction in cats; however, LRS was more efficient in restoring acid-base and electrolyte imbalances (Cunha et al, 2010). If the animal has concurrent hyponatremia, 5% dextrose solution (D5W) and half-strength saline should be avoided. Fluid correction, especially with LRS, should be performed after the obstruction is relieved and should occur over 4 to 6 hours. Hyperkalemia from uroabdomen responds well to abdominal drainage plus intravenous fluid therapy. Hyperkalemia caused by urethral obstruction responds well to intravenous fluids plus elimination of the obstruction. Although seldom required, life-threatening hyperkalemia may be treated with sodium bicarbonate. Bicarbonate therapy drives potassium into cells in exchange for hydrogen ions. Patients with life-threatening hyperkalemia are often moribund and poorly responsive. Additionally, these patients may have respiratory acidosis associated with poor ventilation. Moribund patients should be intubated immediately and hyperventilated to correct respiratory acidosis and improve hyperkalemia. Similar to administering bicarbonate, hyperventilating the patient raises pH and drives potassium intracellularly. Alternatively, life-threatening hyperkalemia can be treated with insulin and dextrose administration (see Box 26-1). Insulin facilitates cellular uptake of potassium, whereas dextrose prevents hypoglycemia following insulin administration. If the hyperkalemia appears immediately life threatening, 10% calcium gluconate given slowly intravenously may protect the heart until other therapy lowers the plasma potassium concentration. Urethral trauma (e.g., gunshot or bite wounds, rupture caused by vehicular trauma, obstruction with stones) or neoplasia may result in urinary obstruction. If the prostatic or penile urethra is torn, subcutaneous urine leakage may occur. Spontaneous rupture of the urethra is uncommon but may occur in dogs (Fig. 26-2). Initial signs of subcutaneous urine leakage are bruising and/or swelling, especially of the inguinal tissue of male dogs. The skin and subcutaneous tissue can necrose if left untreated. Management of patients with urethral rupture before surgery may necessitate placement of an indwelling urinary catheter and/or cutaneous urinary diversion (tube cystostomy; see p. 743). Patients with acute obstruction are oftentimes otherwise healthy (Table 26-1). They may be presented early or late in the course of the urinary obstruction; thus clinical signs may range from a cat that is uncomfortable and straining, but has a normal serum biochemical profile, to a recumbent and very ill acute renal failure patient. In the cat with relatively normal blood work, IV fluids should be limited before the urinary obstruction is relieved. In the obstructed patient in acute renal failure that has major sodium and potassium disturbances with possibly life-threatening electrocardiographic (ECG) changes due to hyperkalemia, hypovolemia, hypotension, weakness, and/or hypothermia, only limited doses of drugs typically can be tolerated without causing further sedation or hypotension (Table 26-2). In the depressed or recumbent obstructed patient, blood work is extremely important in determining electrolyte status and in anticipating the patient’s response to anesthesia. Anesthetic Considerations in the Uncompromised Feline Obstructed Patient *May cause hyperthermia in cats. Anesthetic Considerations in the Compromised Obstructed Patient *Monitor for hyperthermia in cats. Electrolyte (i.e., hyperkalemia) abnormalities and acidosis in patients with urinary obstruction or leakage should be corrected before anesthetic induction (see previous discussion and pp. 756 and 758). Fluids are given intravenously to restore hydration and combat postobstruction diuresis; relief of obstruction without appropriate parenteral fluids can result in hypovolemia and possibly death. An electrocardiogram should be monitored before, during, and after surgery for cardiac arrhythmias. If the animal is hyperkalemic (potassium >7 mEq/L), 0.9% saline should be used for fluid therapy. If serum potassium is normal, a balanced electrolyte solution should be administered. Anticholinergics are not routinely recommended as premedication for trauma patients because they may increase heart rate and oxygen consumption, and may cause a predisposition to cardiac arrhythmias (Table 26-3). If analgesia is needed, butorphanol, hydromorphone, or buprenorphine may be given in small, incremental doses (see Table 12-3 on p. 141). Acetylpromazine is not recommended as a routine premedication and should be used only if volume replacement has been adequate and if shock or severe blood loss is unlikely. However, acetylpromazine can be used to treat obstructed cats with FIC that are not being anesthetized or catheterized (see page 778). Thiobarbiturates are arrhythmogenic and should be used cautiously in animals with preexisting arrhythmias. Combinations of opioids and benzodiazepines (e.g., diazepam) do not cause severe vasodilation or myocardial depression and are useful for inducing anesthesia despite hypovolemia (see Tables 26-2 and 26-3). Etomidate may be used for induction because it maintains cardiovascular stability and is not arrhythmogenic. Alternatively, thiopental or propofol may be administered at reduced dosages, or mask or chamber induction may be considered if the patient is too fractious for immediate intravenous access to be obtained and is not vomiting. Perioperative antibiotic therapy should be considered in animals with urinary obstruction or leakage because infection prolongs healing and promotes stricture formation. Animals with cystic or urethral calculi often have concurrent infections and should be treated with appropriate antibiotics based on urine culture and susceptibility, or antibiotics can be withheld until intraoperative cultures are taken. When culture of urine obtained by cystocentesis is negative, aerobic cultures of a bladder mucosal biopsy and/or cystic calculi should be obtained. Escherichia coli has been found to be the most common isolate in dogs with recurrent or persistent urinary tract infections; however, mixed bacterial infections are common. Potentially nephrotoxic antibiotics (e.g., aminoglycosides) should be avoided in patients with urinary obstruction or possible pyelonephritis (see p. 709). The urinary bladder location varies depending on the amount of urine it currently contains; when empty, it lies primarily within the pelvic cavity. In a 12 kg dog, the bladder holds up to 120 ml of urine without becoming overly distended. The bladder is divided into the trigone, which connects it to the urethra, and the body. The bladder receives its blood supply from the cranial and caudal vesical arteries, which are branches of the umbilical and urogenital arteries, respectively. Sympathetic innervation is from the hypogastric nerves, whereas parasympathetic innervation is via the pelvic nerve. The pudendal nerve supplies somatic innervation to the external bladder sphincter and striated musculature of the urethra (see p. 745). The urethra in male dogs and cats is divided into prostatic, membranous (pelvic), and penile portions (Fig. 26-3). For the bladder, an abdominal incision is made from the umbilicus caudal to the pubis (Fig. 26-4). The proximal urethra (i.e., prostatic urethra) can be reached by this approach; however, pelvic osteotomy (see p. 745) or symphysiotomy is required for adequate exposure of the membranous urethra (i.e., from the caudal edge of the prostate to the ischial arch; Figs. 26-5 and 26-6). The penile urethra begins at the ischial arch and extends to the external urethral penile orifice. The penile urethra may be approached in the perineal (perineal urethrotomy) or scrotal (scrotal urethrotomy) region, or between the scrotum and the external urethral orifice (prescrotal urethrotomy). The skin overlying the site is prepared for aseptic surgery in standard fashion before either approach is used. If the prepuce is to be left in the surgical field, a preputial flush with chlorhexidine or dilute Betadine should be part of the surgical preparation (see p. 40). FIG 26-5 The urethra of male dogs is composed of prostatic, membranous (pelvic), and penile portions. Cystotomy may be performed for removal of cystic and urethral calculi (see p. 759), identification and biopsy of masses (see pp. 767-768), repair of ectopic ureters (see p. 723), or evaluation of urinary tract infection resistant to treatment. The longitudinal incision generally is made on the ventral or dorsal surface of the body of the bladder, away from the urethra; however, ventral exposure is often preferred owing to ease of access and should be performed if identification or catheterization of ureteral openings is necessary. The goal of cystotomy closure is to obtain a watertight seal that will not promote formation of calculi. This has traditionally been accomplished using a single- or double-layer appositional pattern, or by inverting suture patterns using absorbable suture material. A single-layer appositional closure is sufficient if the bladder wall is thick. Even in normal bladders, a single-layer appositional suture pattern (simple continuous [preferred] or simple interrupted) is typically adequate. Luminal penetration is common in thin-walled bladders, but this is not believed to be associated with formation of calculus if absorbable monofilament suture is used. If hemorrhage is expected to be severe, suturing the bladder mucosa as a separate layer (in a simple continuous suture pattern) may be considered to decrease postoperative bleeding (see p. 755 for a discussion of suture materials). Isolate the bladder from the rest of the abdominal cavity by placing moistened laparotomy pads beneath it. Place stay sutures on the bladder apex and trigone to facilitate manipulation (Fig. 26-7, A). Make a longitudinal incision in the ventral or dorsal aspect of the bladder, away from the ureters and urethra, and between major blood vessels. Remove urine by suction or perform intraoperative cystocentesis before cystotomy if suction is not available. Excise a small section of the bladder wall adjacent to the incision to submit for aerobic culture. Check the bladder apex for a diverticulum, and excise it if necessary. Examine the mucosa for defects, and pass a catheter down the urethra to check for patency. Close the bladder in a single layer using a continuous suture pattern with absorbable suture material (see previous discussion). For a two-layer closure, suture the seromuscular layers with two continuous inverting suture lines (e.g., Cushing, followed by Lembert; Fig. 26-7, B). If the dog has severe bleeding tendencies, consider suturing the mucosa as a separate layer with a simple continuous suture pattern. Temporary cystostomy or prepubic catheterization is performed to provide cutaneous urinary diversion in animals with urinary obstruction, or with traumatized or surgically repaired urethras. It also may be advisable for animals with bladder atony secondary to neurologic disease or for prevention of overdistention of the bladder after surgery. Cystostomy may be performed by placing a Foley catheter (6 to 20 French [Fr]; depending on the size of the animal) via a small midline abdominal incision, through a muscle-splitting inguinal approach (Bray et al, 2009), by laparoscopy (Zhang et al, 2010), or percutaneously. Stamey Malecot catheters (10 to 14 Fr) are used for percutaneous placement; however, premature removal of this catheter may occur. Therefore surgically placed Foley catheters are often preferred for long-term catheterization in ambulatory patients. Use of low-profile gastrostomy tubes for cystostomy in dogs and cats has been described and appears to be well tolerated. Regular gastrostomy (Pezzer; see p. 107) tubes have also been used for prepubic catheters. All catheter types could be placed using local analgesia with or without sedation depending on the stability of the animal; however, surgical placement is preferred. The Stamey Malecot catheter can be removed by gentle traction within 3 or 4 days after placement without risk of urinary leakage; however, it is recommended that a Foley catheter be left in for 5 to 7 days. To place a Foley catheter (Fig. 26-8, A), make a small midline incision caudal to the umbilicus in females or adjacent to the prepuce in males. Alternatively, consider a 2 to 3 cm oblique inguinal approach directly over the bladder. Locate the bladder, and place stay sutures and a purse-string suture into the bladder wall (Fig. 26-9, A). Place the tip of the Foley catheter into the abdominal cavity through a separate stab incision in the abdominal wall (Fig. 26-9, B). Make a small stab incision into the bladder (within the purse-string suture), and place the Foley catheter into the bladder lumen. Inflate the balloon with saline, and secure the catheter within the lumen by tying the purse-string suture (Fig. 26-9, C). Pexy the bladder to the body wall with several interrupted absorbable sutures (Fig. 26-9, D). Close the initial incision, and secure the catheter to the skin using a Chinese finger-trap suture (see Fig. 31-10 on p. 999). FIG 26-9 A, To place a Foley catheter, make a small incision and locate the bladder. Place stay sutures and a purse-string suture in the bladder. Place the tip of the Foley catheter into the abdominal cavity through a separate stab incision in the abdominal wall. B, Make a small stab incision into the bladder, and place the Foley catheter into the bladder lumen. C, Inflate the balloon with saline, and secure the catheter within the lumen by tying the purse-string suture around it with a Roman sandal suture (see Fig. 31-10 on p. 999). D, Tack the bladder to the body wall with several absorbable sutures. For a Stamey Malecot catheter (see Fig. 26-8, B), place the dog in right- or left-lateral recumbency and prepare the ventrolateral aspect of the caudal abdominal wall. Do not evacuate the bladder before catheter placement. Make a small skin incision over the bladder, and with the stylet securely fixed within the catheter (with the Malecot wings twisted flat), direct it through the stab incision. Thrust the catheter into the bladder lumen, making sure that the entire flanged portion of the catheter is within the bladder lumen (once urine is obtained, advance the catheter 1 cm farther). Release the Luer-Lok to open the Malecot wings, and remove the obturator. Secure the catheter to the skin. Perform a caudal ventral midline abdominal incision and, if necessary, a pubic symphysiotomy or bilateral pubic and ischial osteotomy (see below). Locate the transected ends of the urethra and débride them. Minimize dissection around the urethra and bladder to prevent damage to the vascular or nerve supply to these structures (Fig. 26-10). Suture the ends with six to eight absorbable interrupted sutures over a transurethral catheter (preferably a Foley catheter or other soft catheter). Leave the catheter in place for 7 to 10 days. If the urethral tissues do not hold suture because of prolonged urine extravasation and subsequent tissue devitalization, delayed repair is indicated. Place a transurethral catheter to divert urine flow for 5 to 7 days. If a catheter cannot be placed from the penile orifice into the bladder, pass a catheter from the bladder into the traumatized tissue, tie it to a catheter placed from the penile urethral orifice, and use it to pull the penile catheter into the bladder. If the urethra does not heal completely in 7 to 10 days, or if stricture occurs, resect the urethral ends and suture them over a catheter, as described for primary repair. Adequate urethral exposure can be obtained in some dogs by splitting the pubic symphysis at the midline. In other dogs, the cranial aspect of the pubis needs to be removed. Bilateral pubic and ischial osteotomy allows exposure of the entire urogenital tract in female dogs. Make a ventral midline incision from the umbilicus to the vulva. Perform a celiotomy from the umbilicus to the pubis, then sharply separate the adductor muscles on the midline of the pubis and ischium. Subperiosteally elevate the adductor muscles until the obturator nerves and half of the obturator foramen are exposed (Fig. 26-11, A). Transect the prepubic tendon along the left pubis to the proposed pubic osteotomy site. Predrill holes in the pubis and ischium on both sides of the four proposed osteotomy sites and craniocaudally along the left pubis (Fig. 26-11, B). Osteotomize the pubis, and elevate the internal obturator muscle from the left pubis and ischium, allowing reflection of the entire central bony plate to the right (Fig. 26-11, C). To close the osteotomy sites, preplace orthopedic wire through the previously drilled holes on the right side. Then, before replacing the bone plate, place sutures through the line of holes in the left pubis and ischium, through the left internal obturator muscle, and back through the adjacent holes in the pubis or ischium. Place orthopedic wire through the left osteotomy sites, then secure the preplaced wires and sutures (Fig. 26-11, D). Reappose the adductor muscles and the prepubic tendon before closing the linea alba. Urethrotomy is performed in male dogs to remove urethral calculi that cannot be retrohydropropulsed into the bladder (see p. 762) and to facilitate placement of catheters into the bladder. Occasionally, urethrotomy is performed for a biopsy of obstructive lesions (i.e., strictures, scar tissue, and neoplasms). Prescrotal or perineal urethrotomy may be performed depending on the level of the obstructive lesion. Prescrotal urethrotomy (Fig. 26-12) is used to remove calculi from the distal penile urethra in dogs, or to place Foley catheters into the urinary bladder if the catheter is of sufficient length and if the obstruction is distal to the proposed urethrotomy incision. Occasionally, urethrotomy can be performed under local anesthesia with opioid sedation in severely depressed or uremic patients. Prescrotal urethrotomies can be left to heal by secondary intention; however, hemorrhage should be expected from the surgical site for 3 to 5 days (particularly during urination). Primary closure is preferred if the mucosa is healthy and if adequate apposition of the urethral mucosa can be achieved, because this decreases postoperative bleeding. With the dog in dorsal recumbency, place a sterile catheter into the penile urethra to the scrotum or to the obstruction. Make a ventral midline incision through the skin and subcutaneous tissue between the caudal aspect of the os penis and the scrotum. Identify, mobilize, and retract the retractor penis muscle laterally to expose the urethra (Fig. 26-13). Using a No. 11 or 15 scalpel blade, make an incision into the urethral lumen over the catheter (Fig. 26-14). Use iris scissors to extend the incision, if necessary. Remove calculi with forceps, and gently flush the urethra with warm saline. Leave the incision to heal by secondary intention, or preferably close the urethra with simple interrupted or continuous appositional absorbable sutures (4-0 or 5-0). Place the first layer in the urethral mucosa and corpus spongiosum, then appose subcutaneous tissue and skin with simple interrupted sutures or a continuous subcuticular suture pattern. FIG 26-14 Use a No. 11 or 15 scalpel blade to make an incision into the urethral lumen over the catheter. Remove the urinary catheter following surgery, regardless of whether the urethra is sutured. Perineal urethrotomy (Fig. 26-15) is occasionally used to remove calculi lodged at the ischial arch and to place catheters into the bladder of large male dogs. Perineal urethrotomy is less commonly indicated than urethrotomy at other sites. This urethrotomy site should be closed to prevent subcutaneous urine leakage. Place a purse-string suture in the anus. Place a sterile catheter into the urethra to the level of the bladder or the site of the obstruction. With the dog in sternal recumbency and the rear limbs hanging over the edge of the table, make a midline incision over the urethra, midway between the scrotum and the anus. Identify the retractor penis muscle, elevate it, and retract it (Fig. 26-16, A). Separate the paired bulbospongiosus muscles at their raphe to expose the corpus spongiosum, then incise the corpus spongiosum to enter the urethral lumen (Fig. 26-16, B and C). Close the incision as just described for prescrotal urethrotomy (Fig. 26-16, D). Prescrotal urethrostomy is performed similarly to prescrotal urethrotomy, except that the urethral mucosa is sutured to the skin. Make a 3- to 4-cm incision in the urethral mucosa as described on p. 746. The length of the urethral incision should be six to eight times its luminal diameter. Periurethral sutures can be placed to the subcutaneous tissue using a simple continuous suture pattern of absorbable suture material. Place simple interrupted absorbable sutures (3-0 to 5-0) from the urethral mucosa to the skin, beginning at the caudal aspect of the incision. Suture the remainder of the urethral mucosa to the skin with simple interrupted or simple continuous sutures (Fig. 26-17). Suture skin at either end of the incision with simple interrupted sutures. Scrotal urethrostomy (Fig. 26-18) is preferred over perineal or prepubic urethrostomy because the urethra is wider and more superficial and is surrounded by less cavernous tissue here than at other sites. Therefore postoperative hemorrhage often is less than with other techniques, and stricture is less likely. If the dog is intact, castrate him and excise the scrotum; otherwise, perform a scrotal ablation (Fig. 26-19, A). Place a sterile catheter into the urethra to the level of the ischial arch or beyond. Make a midline incision over the urethra through the subcutaneous tissue. Identify the retractor penis muscle, mobilize it, and retract it laterally to expose the urethra. Using a No. 11 or 15 scalpel blade, make a 3- to 4-cm incision into the urethral lumen over the catheter (Fig. 26-19, B). Suture the urethra as described on p. 747 for prescrotal urethrostomy (Fig. 26-19, C). Make a 4- to 6-cm incision in skin and overlying tissue, and incise the perineal urethra as described for perineal urethrotomy. The urethral incision should be 1.5 to 2 cm in length. Suture the urethral mucosa to the skin, as described for prescrotal urethrostomy (Fig. 26-20). Place a purse-string suture in the anus, and catheterize the penis if possible. Make an elliptical incision around the scrotum and prepuce, and excise them. Place an Allis tissue forceps on the end of the prepuce or around the catheter to help manipulate the penis. Free the penis and the distal urethra from the surrounding tissue on either side (Fig. 26-21, A). Extend the dissection ventrally and laterally toward the penile attachments at the ischial arch. Elevate the penis dorsally, and sharply sever the ventral penile ligament. Then, transect the ischiocavernosus muscles (Fig. 26-21, B) and the ischiourethralis muscles at their insertion on the ischium to avoid damaging branches of the pudendal nerves and to minimize hemorrhage. Reflect the penis ventrally to expose the dorsal surface. Locate the bulbourethral glands proximal and dorsal to the bulbospongiosus muscle and cranial to the severed ischiocavernosus and ischiourethralis muscles (Fig. 26-21, C). Avoid excessive dorsal dissection to prevent damage to nerves and vessels supplying the urethral muscle. Elevate and remove the retractor penis muscle over the urethra, and longitudinally incise the penile urethra using a No. 11 blade or sharp tenotomy scissors. Continue the urethral incision proximal to the pelvic urethra approximately 1 cm beyond the level of the bulbourethral glands. Pass a closed Halsted mosquito hemostat up the urethra to ensure that the urethral width is adequate. The hemostat should be able to be passed to the level of the boxlocks without resistance. Suture the urethral mucosa to the skin using 4-0 or 5-0 absorbable (polydioxanone [PDS] or polyglyconate [Maxon]) or nonabsorbable (nylon [e.g., Monosof], polypropylene [Prolene]) suture on a taper-cut, swaged-on needle with a simple interrupted or a simple continuous suture pattern. Be sure to suture the urethral mucosa to the skin (it is sometimes difficult to identify mucosa). First, place the most proximal sutures at a 45 degree angle to the skin, then place the remainder (Fig. 26-21, D). Suture the proximal two-thirds of the penile urethra to the skin, and amputate the distal end by placing a horizontal mattress suture through the skin and penile tissues and by severing the penis distal to this ligature. Close the remaining skin with simple interrupted sutures (Fig. 26-22). A modified technique using preputial mucosa has been described, with reported advantages of more rapid return of urination and improved esthetic appearance, as well as risk of postsurgical stenosis caused by hair ingrowth (Yeh and Chin, 2000). The initial approach is as described for a conventional perineal urethrostomy. However, the prepuce is retained and the preputial mucosa is sutured to the penile urethral mucosa, The remainder of the procedure is performed as described previously. Make a ventral midline incision from the umbilicus to the pubis. Free the intrapelvic urethra from the pelvic floor using blunt dissection. Be sure to preserve the urethral artery and its branches. Sever the distal aspect of the intrapelvic urethra. It may be necessary to carefully dissect the prostate from the urethra to ensure that ample urethra is available to exteriorize in some male dogs. Preserve the blood supply to the neck of the bladder. In male dogs, exteriorize the urethra through a small stab incision 2 to 3 cm lateral to the prepuce or within the prepuce. In females, exteriorize the urethra through the ventral midline incision or 2 to 3 cm lateral to the linea alba (Fig. 26-23, A). Spatulate the distal end of the urethra to increase the luminal diameter (Fig. 26-23, B), then suture the urethral mucosa to the skin with interrupted sutures of absorbable or nonabsorbable monofilament suture. Be sure that little tension is placed on the urethrostomy site, and that the urethra is not bent sharply. A Foley catheter can be placed into the bladder through the urethrostomy to divert urine during initial healing (i.e., 24 to 48 hours). Perform this procedure similarly to the one described previously, but retract the skin caudally past the brim of the pubis. Expose the medial boundary of the obturator foramen by elevating the adductor muscle and the cranial portion of the gracilis muscle from the periosteum of the pubis. Partially incise the prepubic tendon, and reflect it laterally to expose the pubic rami (Fig. 26-24). Osteotomize the pubic rami 1.5 cm lateral to the pubic symphysis. Make a transverse incision through the body of the pubic bone and across the pubic symphysis. Rotate the pubic flap ventrally to visualize the intrapelvic urethra. Transect the urethra cranial to the lesion (i.e., stricture) and replace the pubic flap (Fig. 26-25, A). Reappose the muscular aponeuroses of the gracilis and adductor muscles with interrupted or horizontal mattress sutures. Make a 1 cm stab incision 3 cm distal to the caudal extent of the abdominal incision. Tunnel through the subcutaneous tissue and exteriorize the urethra (Fig. 26-25, B). Spatulate the urethral end, and suture it to the skin with 4-0 suture material. Close the abdominal incision, but leave the caudal 1 cm of the linea alba open to prevent crimping of the urethra as it passes over the pubic flap. The strictured perineal urethrostomy site is resected, and the tissues are closed or left open to heal by secondary intention. FIG 26-25 For subpubic urethrostomy, perform a pubic osteotomy (see Fig. 26-24) and rotate the pubic flap ventrally to visualize the intrapelvic urethra. A, Transect the urethra cranial to the lesion and replace the pubic flap. B, Exteriorize the urethra through a stab incision, spatulate the urethral end, and suture it to the skin. Position the cat in dorsal recumbency with the feet secured to the surgical table in a cranial position. Make a small caudal ventral median celiotomy, and expose the urinary bladder 2 cm cranial to the cranial pubic margin. Make a small incision into the bladder, aspirate urine from it, and lavage the bladder with sterile saline. Pass a 6 French urinary catheter into the bladder, and advance it into the proximal urethra to the obstruction site. Secure the catheter to the bladder wall with a temporary purse-string suture. Excise the scrotum and prepuce using an elliptical skin incision that extends to the cranial margin of the pubis. Extend the penis caudally, and denude its ventral surface. Remove fat to expose the caudal and ventral aspects of the pubic symphysis (Fig. 26-26). Elevate muscles medial to lateral on both sides of the ischium to expose its ventral aspect (approximately 1.5 cm wide and 1.5 cm long). Use bone rongeurs to remove the ischium in a caudocranial direction until an ostectomy area approximately 10 mm wide and 12 mm long has been created. Take care to avoid damage to underlying soft tissue. Palpate the indwelling urethral catheter, and make a ventral longitudinal urethral incision over the catheter from the bulbourethral glands to a point 2 to 3 mm from the cranial margin of the ostectomy. Place simple interrupted 4-0 monofilament sutures from the urethral mucosa to the skin margins. Amputate the portion of the penis distal to the bulbourethral glands. Place additional skin sutures to close the remaining wound. Remove the urinary catheter, and pass it into the urethrostomy to ensure patency. Close the cystotomy and celiotomy incisions routinely. Permanent urinary diversion may be indicated when neoplasia involves the bladder trigone. After cystectomy, the ureters may be anastomosed to an isolated bowel conduit or reservoir, or into the intact colon, jejunum, or ileum (Fig. 26-27). Complications associated with ureteral anastomosis in the bowel include reabsorption of electrolytes and nitrogenous waste products, upper urinary tract infection, and neurologic dysfunction. Azotemia, hyperammonemia, hyperchloremia, and metabolic acidosis are also common after these procedures. Because it is a salvage procedure commonly associated with life-threatening complications, such reconstructions should be avoided whenever possible. Stenting may allow the clinician to avoid this technique. Clients electing to have this procedure performed on their animals should be carefully counseled. Ureterocolonic anastomosis is the most commonly performed technique for permanent urinary diversion. The patient should be fasted for 48 hours, and saline enemas should be given 12 to 24 hours before surgery. Prophylactic antibiotics are given and should be continued for at least 8 weeks after surgery. Excise the bladder and the proximal urethra (1 to 2 cm distal to the suspected area of neoplasia), and ligate and transect the ureters. Dissect the ureters from their retroperitoneal attachments. Determine the length of the ureters, and choose a site for each to be implanted into the colon. Stagger the sites for anastomosis of the right and left ureters, so that they are at different sites in the colon. Express feces from the proposed site of ureteral anastomoses, and place atraumatic forceps on the colon. Make a three-sided seromuscular colonic flap for each ureter (Fig. 26-28, A), then create a 4 mm circular defect in the colonic mucosa with tenotomy scissors (Fig. 26-28, B). Transect the end of the ureter, spatulate it, and tunnel the ureters through the seromuscular flap into the colonic lumen. Suture the ureter to the colonic mucosa with simple interrupted sutures (5-0 or 6-0 absorbable suture material; Fig. 26-28, C). Close the flap over the ureter, but be sure to avoid compromising the ureteral lumen (Fig. 26-28, D). If urethral continuity is not completely disrupted, the urethra can heal by regeneration of urethral mucosa in as little as 7 days. Urine extravasation (particularly if infected) delays wound healing and promotes periurethral fibrosis and stricture. Urinary diversion via a urethral catheter or tube cystostomy is therefore indicated for small urethral lacerations (see p. 743). Differences typically are not noted when an indwelling transurethral catheter, a cystostomy catheter, or a combination of the two is used for urinary diversion after transection and anastomosis of the intrapelvic urethra. When complete transection of the urethra occurs, fibrous tissue proliferation is evident in the gaps between the severed ends. Contraction of the fibrous tissue often leads to stricture and urinary obstruction. Primary anastomosis over an indwelling catheter (or proximal urinary diversion) should be performed to decrease the likelihood of stricture formation. The catheter should be left in place for 3 to 5 days. Absorbable suture materials (e.g., polydioxanone [PDS], polyglyconate [Maxon], polyglycolic acid [Dexon], polyglactin-910 [Vicryl], poliglecaprone 25 [Monocryl], glycomer 631 [Biosyn], polyglytone 6211 [Carposyn]) are preferred for bladder and urethral surgery. Most sutures appear to lose tensile strength faster in alkaline urine (such as that seen with Proteus infections) than in infected acidic urine or sterile urine. Polyglycolic acid, polyglactin 910, and poliglecaprone 25 are rapidly degraded in infected urine; polydioxanone, polyglyconate, and glycomer 631 are acceptable for use in sterile bladders and in those infected with E. coli. However, use of any suture that is degraded via hydrolysis may be risky when the bladder is infected with Proteus spp. (see also p. 66) because monofilament absorbable sutures have been shown to degrade within 7 days in Proteus mirabilis–inoculated urine in vitro.
Surgery of the Bladder and Urethra
Preoperative Management
Anesthesia
![]() TABLE 26-1
TABLE 26-1

![]() TABLE 26-2
TABLE 26-2



Antibiotics
Surgical Anatomy
Surgical Technique
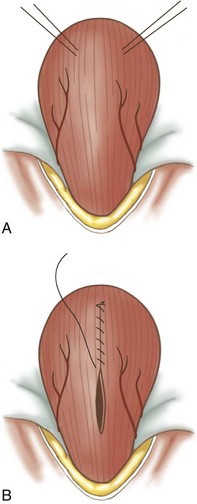
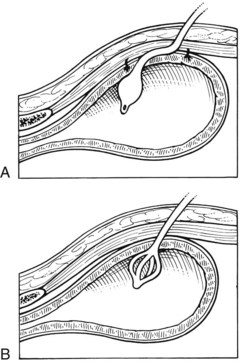
Cystotomy
Cystostomy (Prepubic Catheterization)
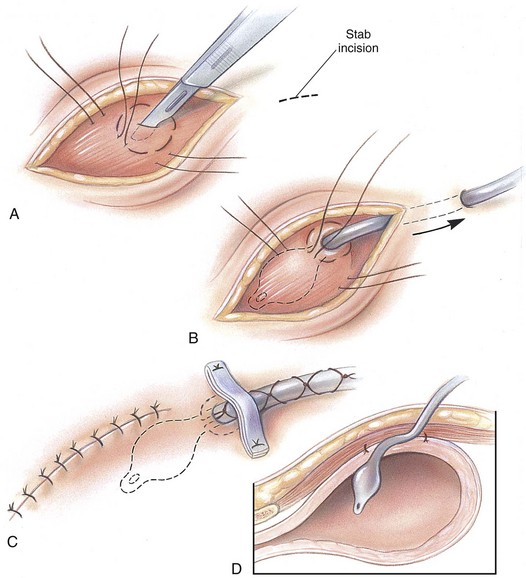
Intrapelvic Urethral Anastomosis
Urethrotomy
Prescrotal urethrotomy
Perineal urethrotomy
Urethrostomy
Prescrotal urethrostomy
Scrotal urethrostomy
Canine perineal urethrostomy
Feline perineal urethrostomy
Prepubic urethrostomy
Subpubic urethrostomy
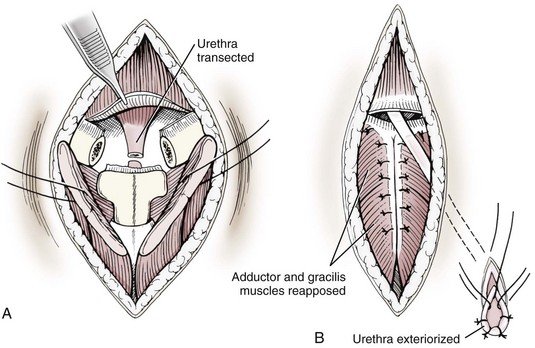
Transpelvic urethrostomy
![]() Permanent Urinary Diversion
Permanent Urinary Diversion
Healing of the Bladder and Urethra
Suture Materials and Special Instruments
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Surgery of the Bladder and Urethra