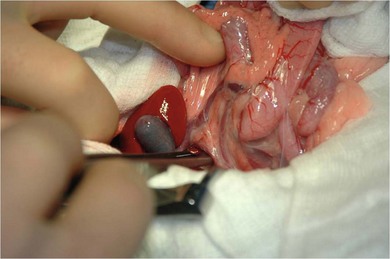
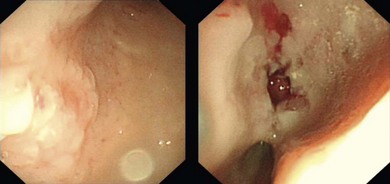
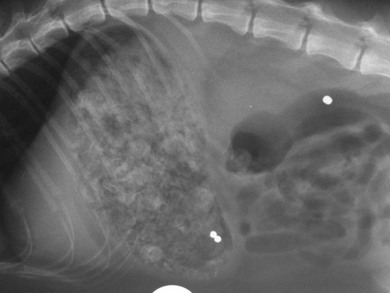
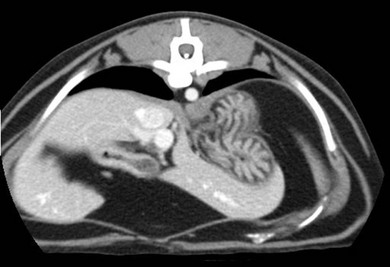
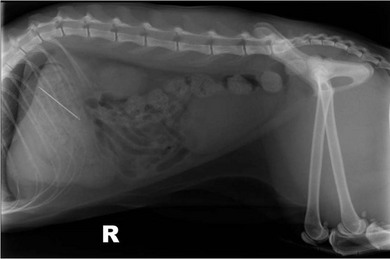
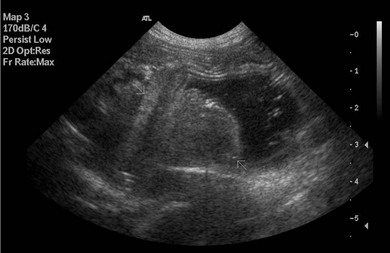
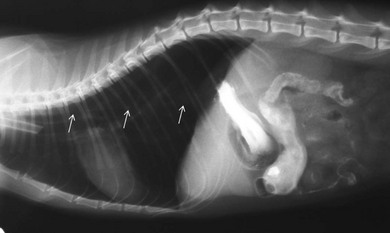
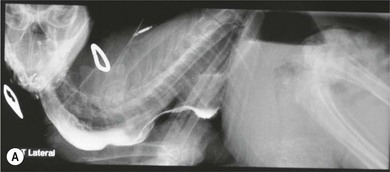
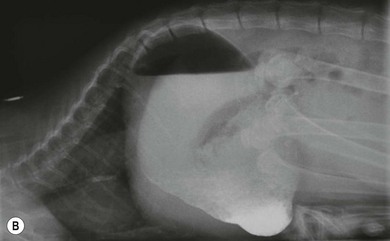
Chapter 28 The stomach is positioned in the cranial abdomen at the level of the costal arch, sitting within the concavity of the visceral surface of the liver (Fig. 28-1). The position of the stomach varies depending upon filling and the size of the surrounding viscera. A cat’s stomach can accept 300–350 mL of liquid.1 Ingesta enters the stomach at the cardia, passing through the lower esophageal sphincter. In the cat this sphincter is comprised of an oblique gastric sling muscle, which lies on the left lateral aspect of the sphincter, and a circular muscle that fully encircles the distal esophagus.2 Ingesta exits the stomach via the pylorus; although the exact mechanisms are not clear, it is evident that the pylorus plays an essential role in coordinating gastric motility and gastric emptying.3 Figure 28-1 A computed tomography scan in transverse section of an adult domestic short-haired cat demonstrating the position of the stomach within the concavity of the visceral surface of the liver. The vascular supply of the stomach is derived from the celiac artery, which branches into the hepatic artery, left gastric artery, and splenic artery. The right and left gastric arteries are situated along the lesser curvature and the right and left gastroepiploic arteries situated along the greater curvature of the stomach. A few branches from the splenic artery (short gastric arteries) supply the fundus. The hepatic artery gives rise to the gastroduodenal artery before continuing as the right gastroepiploic artery. In turn, the gastroduodenal artery supplies the cranial pancreaticoduodenal artery, which is responsible for the vascular supply to the proximal duodenum.4 The parasympathetic innervation is supplied by the vagus nerve and its dorsal and ventral vagal trunks that supply branches to the stomach as they pass through the esophageal hiatus. The sympathetic innervation arises from the splanchnic nerves, with the post-ganglionic fibres from the celiacomesenteric ganglion traveling along the branches of the celiac artery. The feline enteric nervous system is composed of two ganglionated plexuses.5 The gastric lymphatic system drains to the gastric, hepatic, or splenic lymph nodes.1 The gastric lymph nodes are located on the lesser curvature of the stomach within the lesser omentum and are inconsistently present.6 The stomach sits between the two sheets of the greater omentum and these then converge at the lesser curvature of the stomach to form the lesser omentum. The potential space between the visceral and parietal layers of the omentum is called the omental bursa. Access to this space is gained via the epiploic foramen (Fig. 28-2) in the craniodorsal abdomen, or surgically by gently creating a small opening through the greater omentum, into the omental bursa. The left limb of the pancreas is present between the sheets of the visceral layer of the greater omentum adjacent to the dorsal aspect of the stomach and the spleen is situated between the sheets of the parietal layer of the greater omentum. The portion of greater omentum extending between the greater curvature of the stomach and the spleen is referred to as the gastrosplenic ligament. Hematology, serum biochemistry, serum electrolytes, and urinalysis are routinely performed in cats with gastric disease. Gastric ulceration may lead to anemia and serum electrolytes may be deranged in any patient suffering from vomiting. Vomition and loss of chloride, potassium, and acid-rich gastric secretions can lead to hypochloremic hypokalemic metabolic alkalosis; however, acid–base imbalances can be complex and hypovolemia may lead to metabolic acidosis.7 Plain abdominal radiography can be valuable in diagnosis of intraluminal foreign bodies (Fig. 28-3) and gastric distension. In order to identify gastric mucosal or mural lesions using radiography, contrast studies may be necessary. However, ultrasonography is frequently rewarding for evaluating these lesions (Fig. 28-4) and often eliminates the need for contrast studies of the stomach in these patients. Approximately 30% of normal cats have submucosal fat which appears as a radiolucent line within the gastric wall on radiography;8 it is important to note that this is a normal finding. Figure 28-3 A needle foreign body in the stomach of a cat. The cat had ingested a needle and thread and the radiopaque needle is clearly visible. Figure 28-4 An ultrasound image demonstrating a mass involving the cardia of the stomach in a 1-year-old domestic short-haired cat. (Courtesy Alison Moores; © Anderson Moores Veterinary Specialists.) Studies of gastric motility and gastric emptying often rely upon fluoroscopic contrast studies.9 Nuclear scintigraphy is the gold standard for monitoring of gastric transit times and gastric emptying.10 Gastroscopy can be extremely useful in the management of gastric disease. It provides an unrivalled perspective of the gastric lumen and mucosal surface and may reveal lesions eluding other imaging modalities, which could also be missed on external evaluation of the stomach at laparotomy. Endoscopy allows biopsy samples to be obtained for histopathology, thereby providing prognostic information and aiding surgical planning if appropriate (Fig. 28-5). Unfortunately, the mucosal surface of neoplastic lesions may be necrotic or inflamed and histopathology can be misleading.11 Gastroscopy is also a useful technique to allow retrieval of foreign bodies without the need for surgery. However, the withdrawal of very large or sharp objects through the cardia and esophagus should be avoided. Gastric foreign bodies are occasionally seen in the cat, but due to the relative dietary discretion of this species they are far less common than in the dog. The most common gastric foreign bodies include trichobezoars (fur balls) and linear foreign bodies, such as a needle and thread (see Fig. 28-3). Foreign bodies may be responsible for vomiting secondary to gastric outflow obstruction, initiation of gastritis or intestinal plication. They may be seen as incidental findings on abdominal radiographs in the absence of clinical signs. The most common site of anchorage of linear foreign bodies in the cat is under the tongue (Fig. 28-6); however, anchorage at the gastric pylorus is also seen.12 Plication of the duodenum and jejunum occurs as intestinal peristalsis attempts to move the anchored foreign body aborally, resulting in a functional and/or structural intestinal obstruction; however, unlike the dog, secondary intussusception has not been observed.12,13 The radiographic appearance of linear foreign bodies does not conform to the classical picture of dilated loops of small intestine normally associated with intestinal obstruction and could lead to the diagnosis being overlooked. Thorough patient evaluation together with inspection of the abdominal radiographs for bunching of the small intestine, potentially in an abnormal position, and irregular accumulations of small intestinal gas should alert the clinician to the diagnosis. Figure 28-6 Lateral thoracic and abdominal radiograph of a 1-year-old female neutered domestic short-haired cat with a cotton thread linear foreign body lodged under the cat’s tongue. A barium study had previously been performed. The small intestines appear bunched in the cranial abdomen with an irregular contrast pattern. Barium has been absorbed by the cotton thread, highlighting the path of the cotton through the thoracic esophagus from the oral cavity to the abdomen (arrows). Removal of the linear foreign body requires release of the anchorage point, which is commonly at the base of the tongue or the gastric pylorus. Surgical management should be performed promptly following release at the base of the tongue, since loss of intestinal plication may expose areas of intestinal perforation and allow leakage of intestinal contents. When the anchorage site is at the pylorus a combination of gastrotomy and one or more enterotomy incisions may be required. Initially, a gastrotomy is performed in the distal aspect of the gastric body allowing access to the most proximal portion of the foreign body. Gentle traction can be applied to attempt retrieval via the gastrotomy site; however, caution must be exercised to prevent further damage to the mesenteric border of the small intestine. If any resistance is experienced the foreign body must be removed in pieces; the large portion present within the gastric lumen is sectioned from the portion extending into the small intestine allowing the plication effect to be released. The remaining portion of the linear foreign body is removed through additional enterotomy incisions as required and gastrotomy and enterotomy sites are closed routinely. Careful inspection of the mesenteric border of the small intestine is essential to determine if perforation or necrosis of the intestinal wall has occurred. Multiple sites of intestinal resection and anastomosis can be necessary; however, the incidence of intestinal perforation and requirement for intestinal resection anastomosis is far lower in the cat compared to the dog.12,13 It has been theorized that the thinner, less bulky, nature of linear foreign bodies in cats is responsible for a lesser degree of intestinal plication, resulting in a lower morbidity and mortality in this species in comparison to the dog.13 Based upon review of the literature, it is suggested that the probability of septic peritonitis and death secondary to a linear foreign body is approximately half that in a cat in comparison to a dog.12,13 A technique has been described via which linear foreign bodies may be removed through a single enterotomy in the cat.14 The linear foreign body is secured to a red rubber catheter that is inserted into the intestinal lumen and milked towards the anus, together with the intestinal foreign body. Due to the difference in the nature of the foreign bodies they ingest, this technique is unlikely to be applicable to the dog, but it should always be considered in the cat. The authors describe that this technique would be applicable when the anchorage site was within the stomach; however, the catheter would be introduced via the gastrotomy and advanced aborally towards the anus. Conservative management of linear foreign bodies in the cat has been described with successful outcome.15 This involved identifying the anchorage site of the foreign body under the tongue and releasing it, therefore allowing the foreign body to pass. This could be considered if surgical intervention is not possible due to financial constraints; however, due to the risk of gastrointestinal perforation the author would advocate surgical management via exploratory laparotomy. Gastric dysfunction is seen in young, pure bred cats, in particular Siamese cats.16 They are generally less than 6 months old at presentation. The presenting clinical signs include vomiting, which may be projectile, and weight loss; regurgitation may also be present. Investigations reveal gastric distension and a functional delay in gastric outflow associated with pyloric stenosis (Fig. 28-7). Normal gastric emptying should start within 15 minutes of barium ingestion and the stomach is generally empty in four hours.9 Concurrent megaesophagus is often present (Fig. 28-7) and while primary megaesophagus is suspected, it can be unclear if this is due to secondary esophagitis. Postmortem examination of one cat has documented gastric heterotopia, with secretory gastric mucosa within the distended esophagus, presumably contributing to esophagitis.17 Some cats have been successfully managed medically with postural feeding and gastroprotection; however, when delayed gastric emptying or pyloric stenosis are documented, pyloroplasty is warranted. As this appears to be largely a functional obstruction, pyloromyotomy or Y to U pyloroplasty are likely to be sufficient; a Billroth procedure is not warranted. Tube gastrostomy (see Box 28-3) is recommended due to the potential for altered gastric motility following pyloroplasty in a patient where gastric motility may already be abnormal. Figure 28-7 Fluoroscopic barium swallowing study in a 6-month-old male neutered Siamese kitten. (A) The esophagus is moderately dilated along its length. (B) The stomach is distended with food particles, fluid and gas. There is no evidence of barium passing into the small intestines after 15 minutes, suggestive of pyloric stenosis. Gastroduodenal ulceration is occasionally seen in the cat.18–21 Predisposing factors include stress associated with surgery, administration of steroid or non-steroidal anti-inflammatory medication, and as a paraneoplastic syndrome (e.g., systemic effects of mast cell neoplasia or pancreatic gastrinoma).18–21 Several cases reported in the literature have failed to identify an underlying cause. Gastric ulceration and perforation may also occur secondary to primary gastric disease, including neoplasia and inflammatory bowel disease. Clinical signs include inappetence, vomiting, hematemesis, and melena; progression to gastric perforation may result in depression and collapse due to sepsis. Anemia is a common clinical finding in cats with gastrointestinal blood loss; however, it is important to note that hematemesis or melena were present in less than a third of cats with gastrointestinal ulceration in one review.18 Cats that have suffered from gastric perforation may present with depression and abdominal distension due to pneumoperitoneum. Radiographic evidence of marked spontaneous pneumoperitoneum in the cat should raise the suspicion of gastric perforation (Fig. 28-8). Ultrasonographic findings of gastric perforation can be quite non-specific, with bright regional mesenteric fat and peritoneal effusion noted most frequently.22 Abdominal radiography is advocated to facilitate detection of pneumoperitoneum.
Stomach
Surgical anatomy and physiology
Diagnosis and general considerations
Diagnostic imaging
Gastroscopy
Surgical diseases
Gastric foreign bodies
Linear foreign bodies
Pyloric stenosis
Gastroduodenal ulceration and perforation
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Stomach