Rhabdoviridae
Abstract
This chapter describes the properties of rhabdoviruses and features of the diseases they cause in animals.
Keywords
Rhabdovirus; fish; mammals; rabies
The family Rhabdoviridae is ecologically diverse and includes viruses that infect a broad range of hosts, including mammals, birds, fish, insects, and plants. Some rhabdoviruses are transmitted by arthropod vectors. The family Rhabdoviridae contains important pathogens of mammals, notably rabies, vesicular stomatitis, and bovine ephemeral fever viruses, and several economically important viruses of fish. There are also a large number of rhabdoviruses of uncertain pathogenic significance or importance that infect cattle, pigs, marine mammals, marsupials, birds, bats, and reptiles.
Rabies is perhaps the most important zoonosis and one of the oldest and most feared diseases of humans and animals. Rabies was described in ancient Greece by Aristotle, and may have been described even earlier in Egypt. Amongst the most lethal of all infectious diseases, rabies also has the distinction of having stimulated one of the great early discoveries in biomedical science. In 1885, before the nature of viruses was comprehended, Louis Pasteur developed, tested, and applied a rabies vaccine.
Vesicular stomatitis of horses, cattle, and swine has long been recognized as the cause of periodic epizootics of vesicular disease in livestock in the Western hemisphere. The disease was a significant problem in artillery and cavalry horses during the American Civil War. The first large epizootic to be described in detail occurred in 1916, when the disease spread rapidly from Colorado to the east coast of the United States, affecting large numbers of horses and mules and, to a lesser extent, cattle. Bovine ephemeral fever, another important rhabdovirus-induced disease of cattle, was first recognized in Africa in 1867, and is now known to be widespread across Africa, the Middle East, Asia, and Australia.
A number of rhabdoviruses, including infectious hematopoietic necrosis virus, viral hemorrhagic septicemia virus, and spring viremia of carp virus, are the cause of serious losses in the aquaculture industries of North America, Europe, and Asia. It has been proposed that rhabdoviruses of white-beaked dolphin (Lagenorhynchus albirostris) may be derived from the fish on which they feed.
Properties of Rhabdoviruses
Classification
The family Rhabdoviridae is included with the families Bornaviridae, Filoviridae, Pneumoviridae, and Paramyxoviridae in the order Mononegavirales (see Chapter 17: Paramyxoviridae, and Pneumoviridae Fig. 17.1). Rhabdoviruses are enveloped, single-stranded, negative-sense RNA viruses. The family Rhabdoviridae currently includes eleven genera, with additional genera proposed. The increasing taxonomic subdivision of rhabdoviruses is a reflection of their inherent genome plasticity, likely as a result of the discontinuous replication strategy they utilize that leads to remarkable variation in both genome size and organization among these viruses. Pathogenic rhabdoviruses of warm-blooded animals are included in the genera Lyssavirus, Vesiculovirus, and Ephemerovirus, and those of fish in the genera Novirhabdovirus, Perhabdovirus, and Sprivivirus (Table 18.1). The genus Tibrovirus includes viruses that have been isolated from healthy cattle and Culicoides biting midges in Australia, and the genus Tupavirus includes viruses of birds (American coot, Fulica americana), tree shrews (Tupaia belangeri) and, provisionally, a virus that recently was identified in healthy wild and domestic pigs in Japan. A proposed new genus Ledantevirus includes a monophyletic group of viruses with strong ecological association with bats, some of which have also been isolated from humans, rodents and livestock. Two additional genera (Cytorhabdovirus and Nucleorhabdovirus) include viruses that exclusively infect plants, and the genus Sigmavirus includes viruses that infect insects. Individual species of rhabdovirus are distinguished genetically and serologically.
Table 18.1
Rhabdoviruses of Veterinary and Zoonotic Importance
| Genus/Virus | Geographic Distribution |
| Rhabdoviruses of Mammals | |
| Genus Ephemerovirus | |
| Bovine ephemeral fever virus | Asia, Africa, Middle East, Australia |
| Genus Lyssavirus | |
| Rabies virus | Worldwide except Australasia, Antarctica, and certain islands; recently eradicated from portions of Europe and Scandinavia |
| Mokola virus | Africa |
| Lagos bat virus | Africa |
| Duvenhage virus | Africa |
| European bat lyssaviruses 1 and 2 | Europe |
| Australian bat lyssavirus | Australia |
| Genus Vesiculovirus | |
| Vesicular stomatitis Indiana virus | North, Central, and South America |
| Vesicular stomatitis New Jersey virus | North, Central, and South America |
| Vesicular stomatitis Alagoas virus | South America |
| Cocal virus | South America |
| Rhabdoviruses of Fish | |
| Genus Novirhabdovirus | |
| Infectious hematopoietic necrosis virus | North America, Europe, Asia |
| Viral hemorrhagic septicemia virus | Europe, North America, Asia |
| Snakehead virus | Southeast Asia |
| Hirame rhabdovirus | Japan, Korea |
| Genus Sprivivirus | |
| Pike fry rhabdovirus | Europe |
| Spring viremia of carp virus | Widespread |
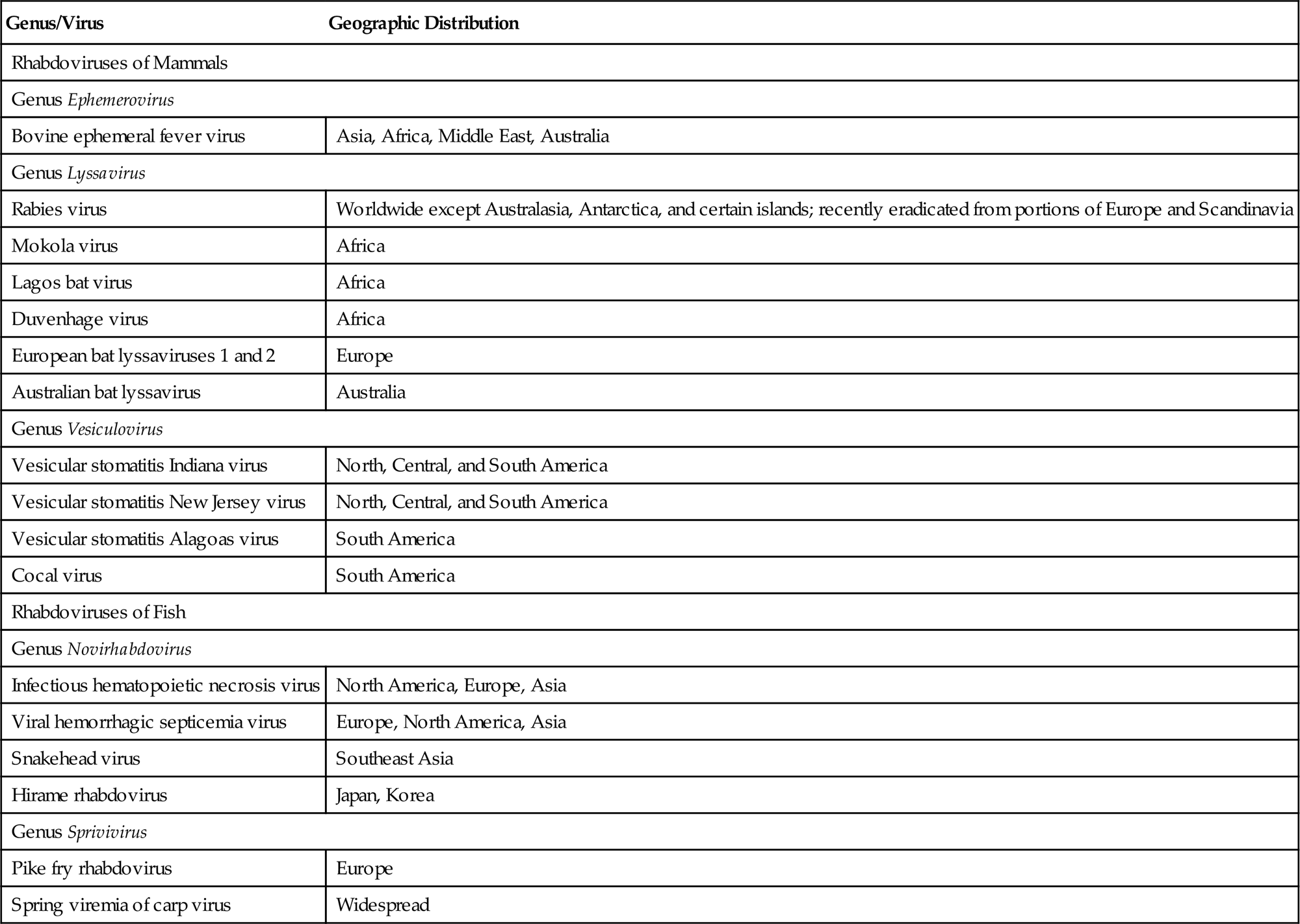
The genus Lyssavirus (from the Greek “Lyssa” meaning the spirit of mad rage) includes rabies virus and closely related viruses, including Mokola, Lagos bat, Duvenhage, European bat lyssaviruses 1 and 2, and Australian bat lyssavirus. Each of these viruses is capable of causing rabies-like disease in animals and humans. Certain terrestrial mammals are reservoir hosts of rabies virus, and bats are potential reservoirs of both rabies and the rabies-like viruses. The genus Vesiculovirus includes vesicular stomatitis Indiana virus, vesicular stomatitis New Jersey virus, vesicular stomatitis Alagoas virus, and Cocal virus, in addition to several similar viruses that also cause vesicular disease in horses, cattle, swine, and humans. The genus Ephemerovirus contains bovine ephemeral fever virus and other serologically distinct viruses that also infect cattle but are typically not pathogenic. The genus Novirhabdovirus contains important pathogens of fish, notably infectious hematopoietic necrosis virus and viral hemorrhagic septicemia virus, the genus Perhabdovirus includes sea trout and perch rhabdoviruses, and the genus Sprivivirus includes spring viremia of carp virus and pike fry rhabdovirus.
Virion Properties
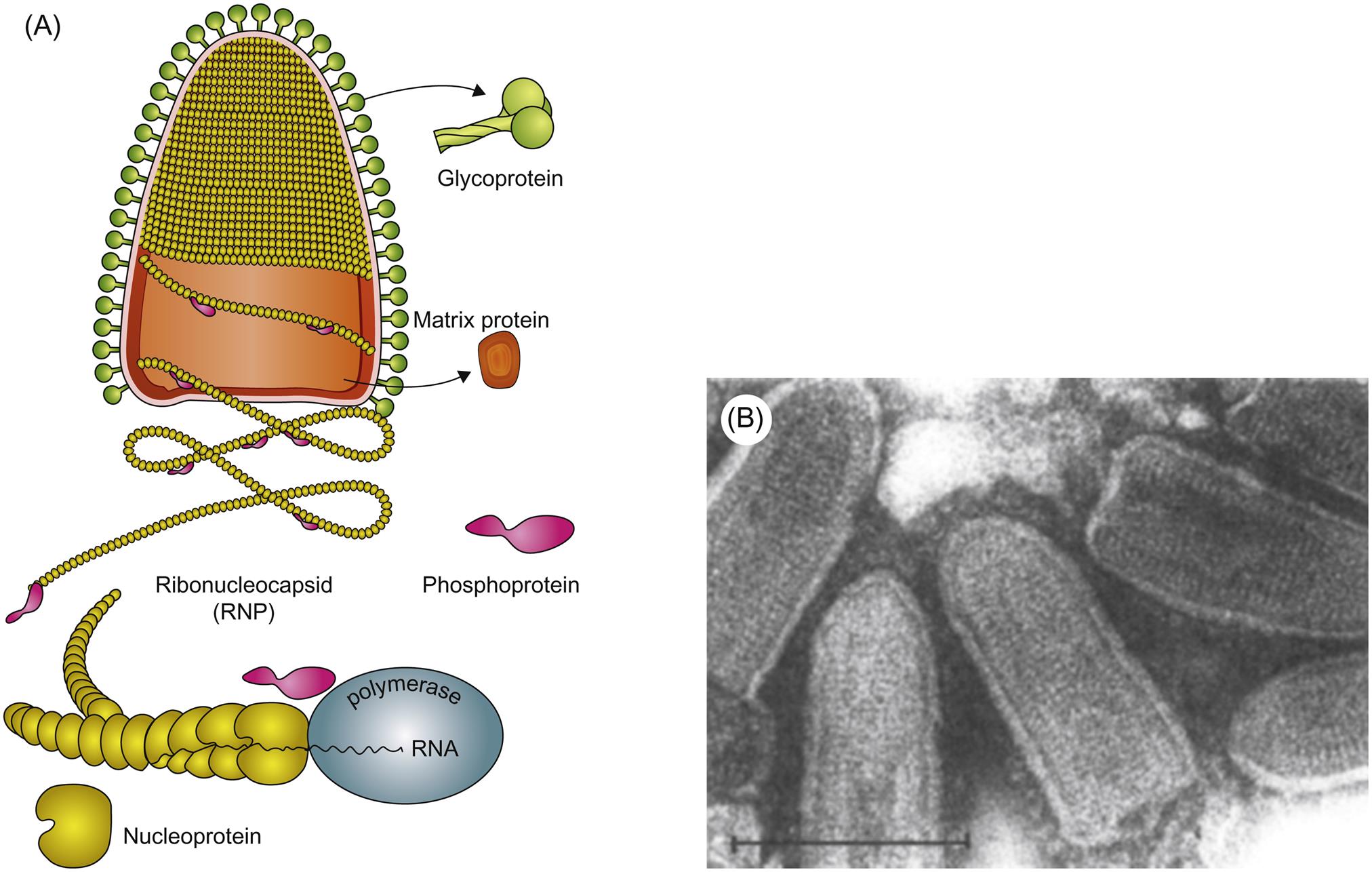
Rhabdovirus virions are approximately 45–100 nm in diameter and 100–430 nm long, and consist of a helically coiled cylindrical nucleocapsid surrounded by an envelope with large (5–10 nm in length) glycoprotein spikes (Table 18.2). The precise cylindrical form of the nucleocapsid is what gives the viruses their distinctive bullet or conical shape (Fig. 18.1). The genome is a single molecule of linear, negative-sense, single-stranded RNA, 11–15 kb in size. For example, the rabies virus (Pasteur strain) genome consists of 11,932 nucleotides that encode five genes in the order 3′-N-P-M-G-L-5′: N is the nucleoprotein gene that encodes the major component of the viral nucleocapsid; P is a phosphoprotein that serves as a cofactor for the viral polymerase; M is an inner virion protein that facilitates virion budding by binding to the nucleocapsid and to the cytoplasmic domain of the glycoprotein; G is the glycoprotein that forms trimers that make up the virion surface spikes; L is the RNA-dependent RNA polymerase that functions in transcription and RNA replication. The glycoprotein (G) contains neutralizing epitopes, which are targets of antibody-mediated immunity (eg, antibodies induced by vaccination); it and the nucleoprotein include epitopes involved in cell-mediated immunity. Virions also contain lipids, their composition reflecting the composition of host-cell membranes, and carbohydrates as side chains on the glycoprotein.
Table 18.2
Virions are enveloped, bullet shaped, 45–100 nm in diameter and 100–430 nm long (although some are longer, some shorter), and consist of an envelope with large spikes within which is a helically coiled cylindrical nucleocapsid
The genome is a single molecule of linear, negative-sense, single-stranded RNA, 11–15 kb in size
Cytoplasmic replication
Viral RNA-dependent RNA polymerase (transcriptase) transcribes five subgenomic mRNAs, which are translated into five proteins: (1) L, the RNA-dependent RNA polymerase (transcriptase); (2) G, the glycoprotein that forms the envelope spikes; (3) N, the nucleoprotein, the protein that associates with RNA to form the viral nucleocapsid; (4) P, a phosphoprotein that mediates binding of L protein to the nucleocapsid; (5) M, which associates with the viral nucleocapsid and lipid envelope
Maturation is by budding through the plasma membrane
Some viruses, such as the vesicular stomatitis viruses, cause rapid cytopathology, whereas others, such as unadapted rabies virus, are noncytopathogenic
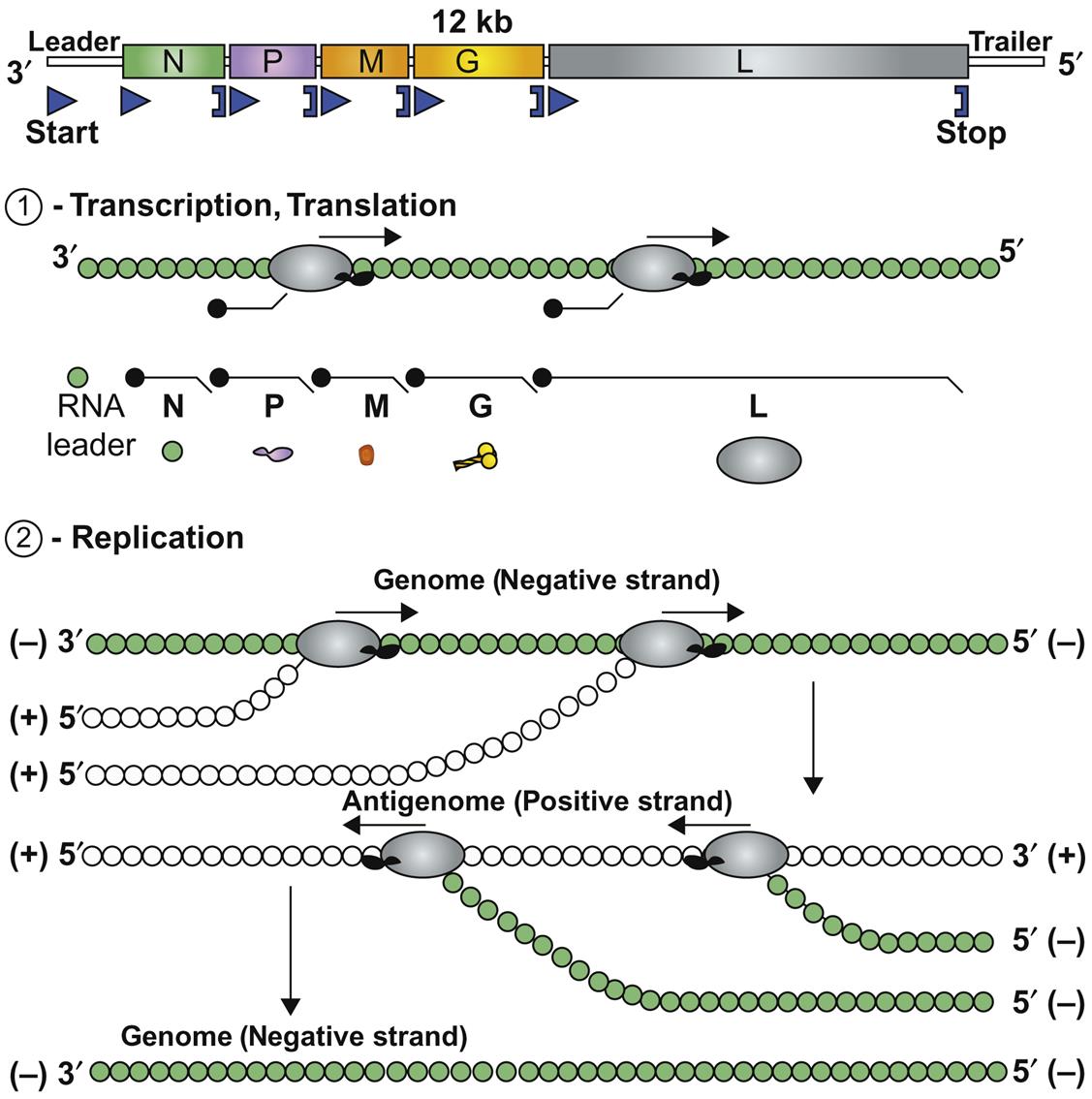
Each open reading frame in the rhabdovirus genome is flanked by conserved transcription initiation and termination sequences that direct transcription of the corresponding mRNAs. Some rhabdoviruses also have additional genes or pseudogenes interposed, either as alternative or overlapping open reading frames within the structural protein genes or as independent open reading frames in the regions between the structural protein genes. This genome plasticity of rhabdoviruses has resulted in the remarkable variation in genome organization and gene expression strategies that likely explains their ecological diversity as a group, and their increasingly complex taxonomic segregation.
Rhabdoviruses are relatively stable in the environment, especially in cool moist environments and when the pH is alkaline—vesicular stomatitis virus can contaminate water troughs for many days for example—but the viruses are thermolabile and sensitive to the ultraviolet irradiation of sunlight. Rabies and vesicular stomatitis viruses are inactivated readily by detergent-based disinfectants, and iodine-containing preparations are commonly applied as disinfectants for reducing or eliminating fish rhabdoviruses such as those that occur on the surface of fish eggs.
Virus Replication
Replication of rhabdoviruses is described and illustrated in Chapter 2, Virus Replication (Fig. 2.8). Virus entry into host cells occurs by receptor-mediated endocytosis via coated pits, and subsequent pH-dependent fusion of the viral envelope with the endosomal membrane releases the viral nucleocapsid into the cytoplasm, where replication exclusively occurs. The viral glycoprotein G is solely responsible for receptor recognition and cell entry. Specific cell receptors have not clearly been identified for individual rhabdoviruses; several apparently nonessential (or redundant) receptors have been identified for rabies virus, including: neurotrophin receptor p75NTR, a member of the tumor necrosis factor receptor family; the muscular form of the nicotinic acetylcholine receptor; neuronal cell adhesion molecule, a member of the immunoglobulin superfamily; and perhaps other components of the cell membrane such as gangliosides. Phosphatidyl choline is a proposed receptor for vesicular stomatitis virus, and fibronectin for viral hemorrhagic septicemia virus.
Replication first involves messenger RNA (mRNA) transcription from the genomic RNA via the virion polymerase (Fig. 18.2). When sufficient quantities of the nucleocapsid (N) and phosphoprotein (P) have been expressed, there is a switch from transcription of mRNA to positive-sense antigenomes, which then serve as the template for synthesis of negative-stranded, genomic RNA. Using virion RNA as a template, the viral transcriptase transcribes five subgenomic mRNA species. There is only a single promoter site, located at the 3′ end of the viral genome; the polymerase attaches to the genomic RNA template at this site and, as it moves along the viral RNA, it encounters stop–start signals at the boundaries of each of the viral genes. Only a fraction of the polymerase molecules move past each junction and continue the transcription process. This mechanism of discontinuous transcription of genomic RNA-called attenuated transcription, or stop–start transcription or stuttering transcription-results in more mRNA being made from genes that are located at the 3′ end of the genome and a gradient of progressively less mRNA from downstream genes N>P>M>G>L. This allows large amounts of the structural proteins such as the nucleocapsid protein to be produced relative to the amount of the L (RNA polymerase) protein.
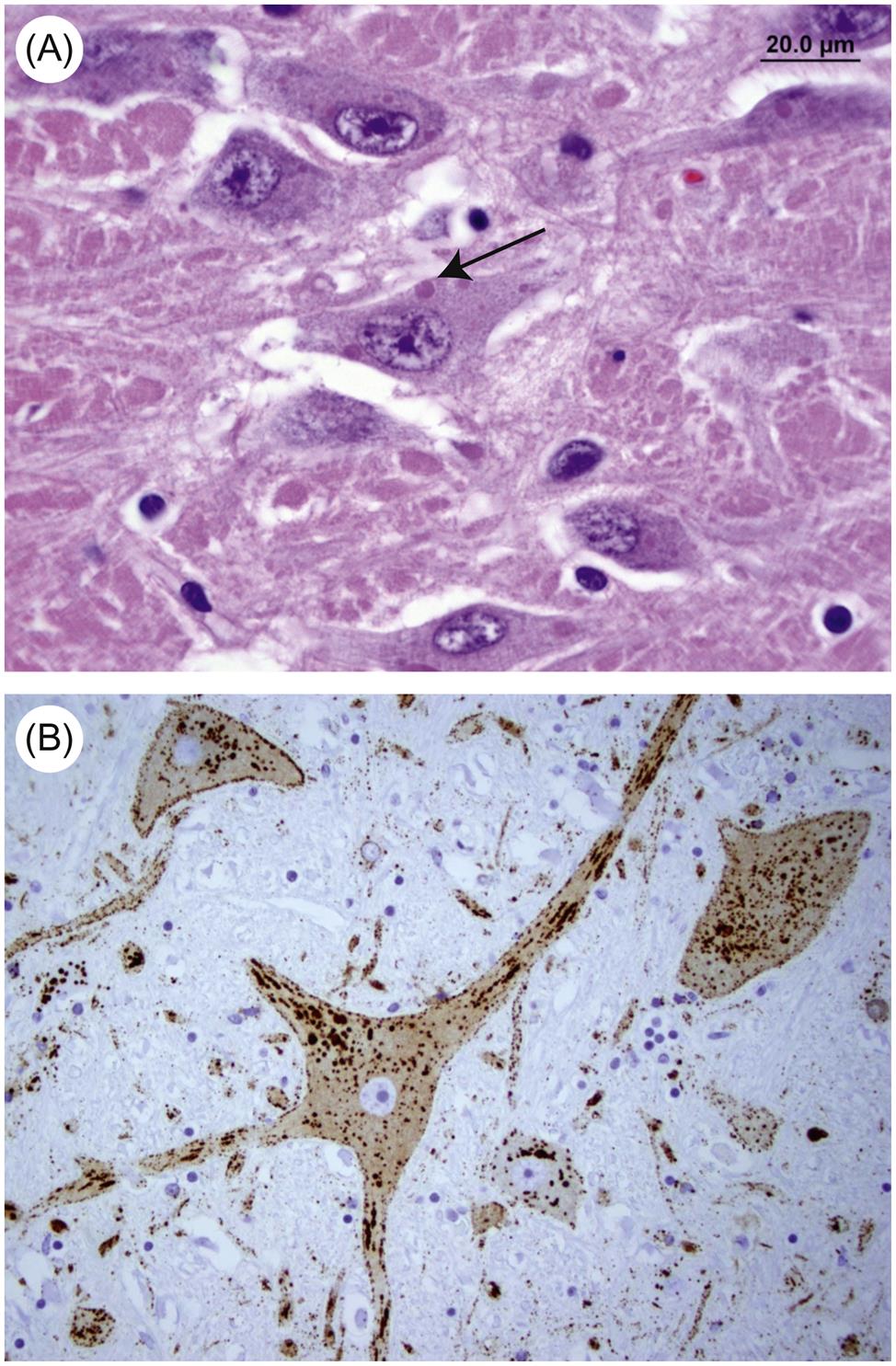
Attachment of nucleocapsid protein to newly formed genomic RNA molecules leads to the self-assembly of helically wound nucleocapsids. Through the action of the matrix protein (M) protein, nucleocapsids are in turn bound to cell membranes at sites where the envelope spike glycoprotein is inserted. The M protein is also translocated to the nucleus of infected cells, and may inhibit host-cell transcription. Virions are formed by the budding of nucleocapsids through cell membranes. Budding of rabies virus occurs principally from intracytoplasmic membranes of infected neurons, whereas the same process occurs almost exclusively on plasma membranes of salivary gland epithelial cells. Vesicular stomatitis virus causes rapid cytopathology in cell culture, whereas the replication of unadapted rabies and bovine ephemeral fever viruses is slower and usually noncytopathic, because these viruses do not shut down host-cell protein and nucleic acid synthesis. Rabies virus produces prominent cytoplasmic inclusion bodies (Negri bodies) in infected cells (Fig. 18.3).
Defective interfering virus particles are commonly formed during rhabdovirus replication (see Chapter 2: Virus Replication). These are complex deletion mutants with substantially truncated genomic RNA, which interfere with normal virus replication processes. Defective interfering particles are proportionately shorter than infectious virions.
Members of the Genus Ephemerovirus
Bovine Ephemeral Fever Virus
Bovine ephemeral fever, also called 3-day stiff-sickness, is an arthropod-transmitted disease of cattle and water buffalo that spans tropical and subtropical zones of Africa, Australia, the Middle East (including Egypt, Israel, Turkey, and Saudi Arabia), and Asia (including Southeast, Central, and Far Eastern Asia). From these enzootic sites the disease extends intermittently into temperate zones, causing epizootics of variable severity. The disease has not been reported in North or South America or in Europe. Several related ephemeroviruses naturally infect cattle and other animals in endemic areas, but typically do not cause severe disease (eg, Adelaide River, Berrimah, Kimberley, and Kotonkan viruses).
Clinical Features and Epidemiology
Clinical signs of bovine ephemeral fever in cattle are characteristic, but all are not seen in an individual animal. Onset is sudden, and the disease is marked by a biphasic or polyphasic fever, with inappetance and an immediate severe drop in milk production. Other clinical signs are associated with the second and later febrile phases; these include depression, stiffness, lameness and drooling of saliva, and—less often—nasal and ocular discharges, cessation of rumination, constipation, and abortion. Infrequently, there is diarrhea and temporary or permanent paresis. Usually, recovery is dramatic and complete in 3 days (range 2–5 days), with the exception of a return of milk production. When cows are infected early in lactation, it may take weeks before they return to normal production though often to levels less than expected. If cows are infected in mid-lactation, they may not return to commercial production. Bulls can be severely affected and show infertility for 3–6 months as a result of the severe fever that accompanies acute infection. Morbidity rates often approach 100%, and the mortality rate in an outbreak is usually very low (less than 1%), but on occasion it can reach 5% in mature, well-conditioned beef cattle and high-producing dairy cattle. Subclinical cases of bovine ephemeral fever virus infection occur, particularly in very young animals, but determination of their prevalence using serological (antibody detection) assays is confounded by intercurrent infections in the same areas by related but nonpathogenic rhabdoviruses.
Clinical disease is restricted to domestic cattle and buffalo, although a variety of other ruminants appear to be susceptible to subclinical infection. In enzootic areas, ephemeral fever is a seasonal disease that occurs in the summer and autumn, especially in the rainy season. Bovine ephemeral fever virus most probably is transmitted by arthropod vectors, although these are yet to be definitively characterized. Potential vectors include culicine and anopheline mosquitoes, and possibly Culicoides midges; both enzootic and epizootic spread is limited by the distribution of appropriate vectors.
Pathogenesis and Pathology
The pathogenesis of the disease is complex and probably reflects pathophysiologic and immunologic effects mediated by the release and activity of various inflammatory mediators (so-called “cytokine storm”). Injury to the endothelial lining of small blood vessels is central to the expression of bovine ephemeral fever, but there is no evidence that the virus causes widespread tissue destruction. In all cases, there is an early neutrophilia with an abnormal level of immature neutrophils in the circulation (left shift). There is an increase in plasma fibrinogen and a significant decrease in plasma calcium. Therapeutically, there is a dramatic response to anti-inflammatory drugs, and often to calcium infusion. Gross (macroscopic) lesions include serofibrinous polyserositis and synovitis, pulmonary and lymph node edema, and focal necrosis of selected muscles.
Diagnosis
Although the clinical and epidemiological presentations of bovine ephemeral fever are highly characteristic, laboratory confirmation is required. A diagnostic increase in antibody titer, or preferably, seroconversion, can be detected by enzyme immunoassay, including blocking ELISA, by neutralization assays, which are virus specific, or by immunofluorescence or agar gel precipitin tests, which are cross-reactive with related rhabdoviruses. Although virus isolation was the traditional “gold standard,” real time RT-PCR assays are now routinely employed to achieve rapid (ie, “same day”) confirmation of bovine ephemeral fever.
Immunity, Prevention, and Control
Infection results in solid, long-lasting immunity. Because outbreaks tend to involve a high proportion of animals in a herd, repeat clinical episodes usually involve young animals born since previous outbreaks. Prevention by vector control is impractical in the areas of the world where this disease is prevalent. Vaccination has been used in endemic countries, including Australia, Bahrain, China, Egypt, Israel, Japan, Namibia, Saudi Arabia, South Africa, South Korea, Taiwan, the Philippines, and Turkey. Both inactivated and live-attenuated vaccines are used, although repeated immunization is often required. Problems with conventional vaccines stem from their lack of potency—inactivated vaccines require more antigenic mass than it has been possible to achieve economically, and live-attenuated vaccines suffer from a loss in immunogenicity linked to the attenuation process (see Chapter 4: Antiviral Immunity and Virus Vaccines). Immunization with the bovine ephemeral fever virus G protein alone can confer protective immunity in cattle, as can recombinant vectors that express this protein.
Members of the Genus Lyssavirus
Rabies Virus
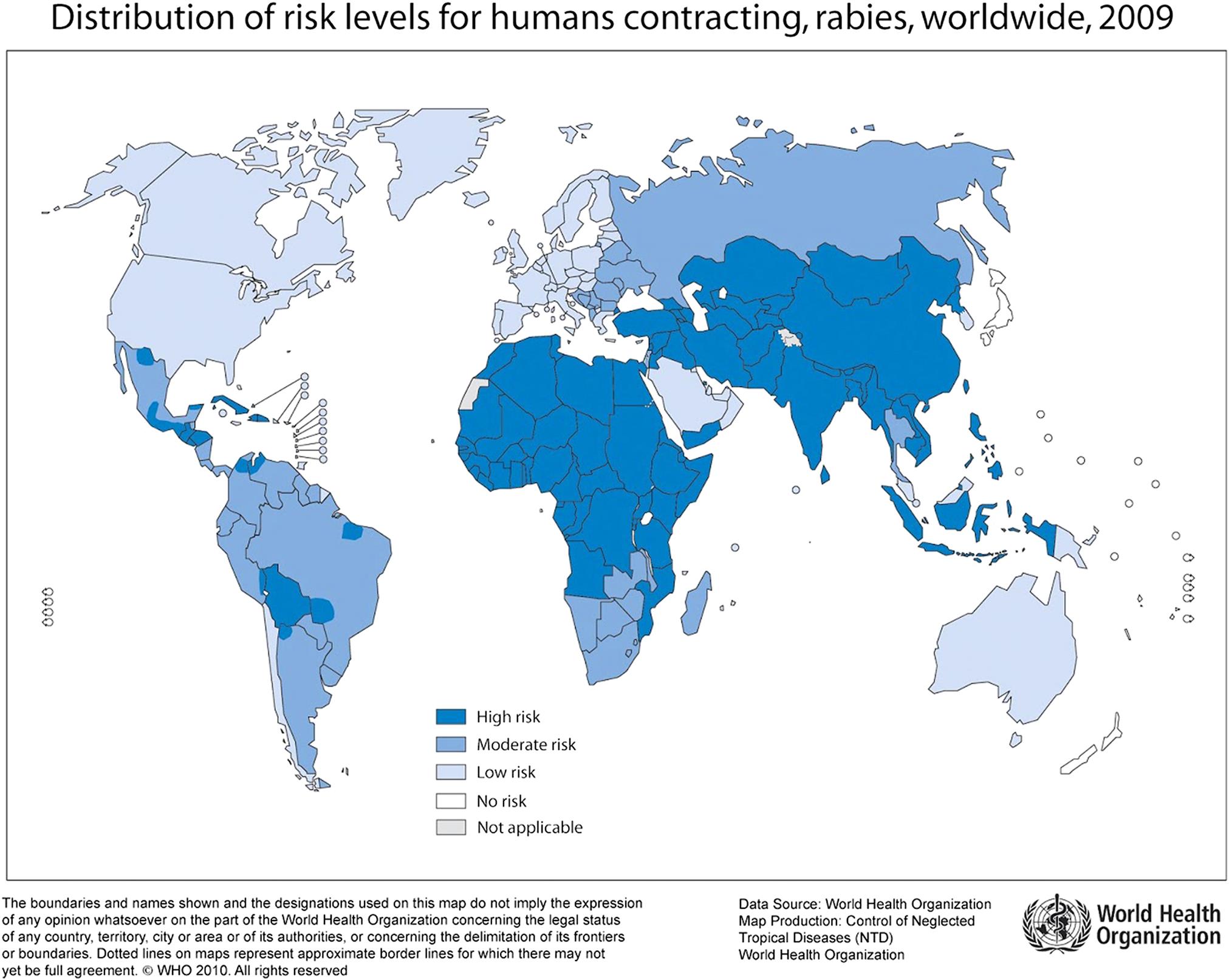
Rabies virus can infect all mammals and infection virtually always results in the death of susceptible (ie, unvaccinated) individuals, unless they are aggressively treated before signs appear. The disease occurs or has occurred throughout extensive portions of the world (Fig. 18.4), although certain regions have never reported domestic rabies (eg, Japan, New Zealand) and others are now considered to be free of terrestrial rabies after wildlife rabies eradication campaigns (eg, Switzerland, France). Complicating the concept of countries having terrestrial rabies-free status are situations in which rabies-related lyssaviruses that are transmitted by bats are enzootic and human and animal deaths from these agents occur (eg, European bat lyssavirus 2 in the United Kingdom and Australian bat lyssavirus in Australia). Rabies is estimated to cause more than 60,000 human deaths worldwide annually, with 40–50% of rabies deaths in children younger than 15 years of age. An estimated 10 million people receive postexposure treatments each year after being exposed to rabies-suspect animals. A large majority of human rabies cases still result from bites of rabid dogs, particularly in Africa and Asia. In contrast, wildlife rabies is now the major threat in North America.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree