CHAPTER 51 Protozoal Abortion in Cattle
Ruminant protozoal abortifacients include Tritrichomonas foetus and Neospora, Toxoplasma, and Sarcocystis spp. The latter three genera all are members of the family Sarcocystidae. The genus Sarcocystis is composed of 130 species of cyst-forming coccidians with differences in life cycle and pathogenicity.1 All Sarcocystidae species with known life cycles exploit natural predator-prey relationships. Sexual reproduction takes place in the intestine of the predator, resulting in fecal passage of oocysts that are orally infective for the prey species (intermediate host); asexual reproduction takes place in tissues of the prey species, which are then orally infective for the predator species (definitive host). For example, the several Sarcocystis species that infect the bovine—Sarcocystis cruzi, Sarcocystis hirsuti, and Sarcocystis hominis—have raccoons and canids (dog, wolf, coyote, fox), felids, and primates (humans, monkey), respectively, as definitive hosts. Felids act as the definitive hosts for Toxoplasma gondii, with almost all warm-blooded animals potentially serving as intermediate hosts. Infection by T. gondii also can be maintained in successive generations of two common intermediate hosts (rats and mice) by congenital (dam-to-daughter) transmission.2,3
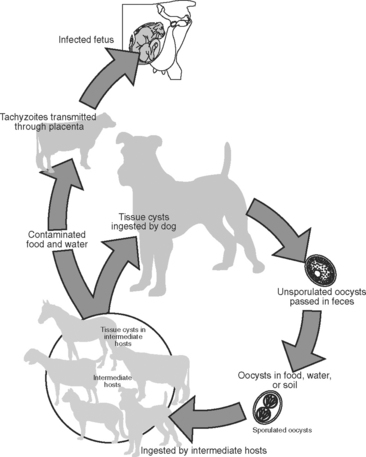
The life cycle of Neospora has only recently been determined but has been shown to follow a pattern similar to that for other species of Sarcocystidae (Fig. 51-1). Oocysts were found in dog feces,4 and it appears that the dog can play the role of both an intermediate and a definitive host. Other intermediate hosts include ruminants and canids.5 This agent is maintained in successive cattle generations by congenital (dam-to-daughter) transmission. It also is increasingly clear that Neospora caninum is the most important abortifacient agent in this group.
NEOSPORA CANINUM
N. caninum was discovered in 1984 as the cause of congenital infection of the central nervous system in dogs, and in 1988 the organism was isolated and named.6 Slight ultrastructural and antigenic differences exist between tissue phases of T. gondii and N. caninum.7,8 Comparison of ribosomal RNA or DNA sequences indicates a close phylogenetic relationship but distinct species in analysis by random amplified polymorphic DNA–polymerase chain reaction.9–11 The definitive host of N. caninum is the dog4; wild canids are intermediate hosts and may be implicated as definitive hosts with further study.12,13 Researchers have found antibodies in naturally exposed water buffalo, coyotes, red foxes, and camels, suggesting that these animals are natural intermediate hosts.5 Congenital infection by N. caninum has been documented in many species. Naturally occurring congenital infections were first documented in dogs14 and cattle.6 Further study suggests that natural and experimental congenital infection occurs in horses, sheep,15 goats,16 deer,17 and mice.18 As has been proved for T. gondii, it is possible that infection is acquired congenitally, by ingestion of oocysts (fecal contamination of feed), or by contact (nasal, ocular, or oral) with cysts or tachyzoites.19
Since the 1960s, there have been many reports of abortion in cattle due to unidentified protozoa, often tentatively assumed to be either Sarcocystis spp. or T. gondii.20 Retrospectively, most cases involved microscopic lesions that are now recognized as virtually diagnostic for fetal infection by N. caninum. These microscopic lesions are most consistently nonsuppurative encephalitis with foci of necrosis and gliosis, and nonsuppurative myositis, hepatitis, and myocarditis. Protozoa are rarely found by microscopic evaluation of hematoxylin-eosin (H&E)-stained sections from affected fetuses.21 With use of immunohistochemistry studies,22 however, protozoa can be visualized in most fetuses with lesions typical of protozoal abortion.7
Reported cases indicate worldwide distribution of this abortifacient in cattle. Cases have been documented in Canada,23,24 the United States,25,26 the Netherlands,27 New Zealand,28 South Africa,29 Australia,30,31 Mexico,32 England and Wales,33 and Japan.34 Continued investigation during the past decade validates the worldwide distribution of infection in dairy and beef cattle globally.5 In California21,35 and similarly in New Zealand,28 Neospora has been diagnosed as the cause of abortion in approximately 20% of fetuses from dairy cattle submitted to diagnostic laboratories and the cause of abortion in approximately 44% of all fetuses originating from California dairy herds with a prior history of Neospora abortions.36 In the midwestern United States, a survey of 655 aborted bovine fetuses submitted to a state diagnostic laboratory revealed 2.7% to be the result of Neospora infection; of interest, 19 of 20 of the Neospora-infected fetuses represented dairy breeds and 12% of submitted fetuses from dairy cows were diagnosed with Neospora infections.37
The dog, as the definitive host of Neospora, contributes to the horizontal transmission of the agent and thereby the establishment of infection.4,38–42 Infection can then be propagated by vertical transmission in the bovine through multiple generations.41–43 This horizontal introduction by a definitive host combined with vertical propagation from the infected intermediate host helps to explain the high prevalence and worldwide distribution of Neospora abortion. Because the dog generally has both worldwide distribution and a common association with dairies and livestock enterprises, the likelihood of cattle exposure to infection may be increased. Of interest, naturally occurring abortions and congenital infection with Neospora also have been documented in sheep,44 goats,16 horses,45 and black-tailed deer.17
Neospora-induced abortions typically occur during the early second trimester25,26,28,35–37 but can occur throughout gestation.28,32,35 On gross examination, aborted fetuses often are moderately or severely autolyzed, but microscopic diagnosis usually is possible because of persistence of both characteristic lesions and protozoa.28,32,33,43 Desirable samples for submission to the diagnostic laboratory include the entire fetus and placenta or samples from the brain, heart and liver, as well as body fluids or blood serum.5 Although autolysis may present some difficulties in preparation of samples, semiliquid brain tissue should be fixed in 10% buffered neutral formalin for histologic and immunohistochemistry examination.5 Cows aborting Neospora-infected fetuses are not clinically sick, and in one report, conception rates to artificial insemination were not adversely affected by a history of Neospora abortion.32
A sensitive and specific serologic test is available,46 and serum from congenitally infected calves is positive before intake of colostrum.6,46 Term calves with serologic and/or microscopic evidence, or both, of Neospora infection have been reported to have enhanced survivorship during the preweaning period, to be essentially clinically normal, or to have various degrees of central nervous system impairment.6,30,47–50 Serologic studies in California drylot dairies reveal that herd infection rates can be estimated by serologic testing of calves before colostral intake, because congenital infection occurs in 78% to 80% of seropositive cows.47 Repeated serologic testing of individual cows from these dairies reveals an apparent lack of new infections (i.e., serologically positive cows remain positive and serologically negative cows remain negative). Furthermore, the percentage of serologically positive cows that abort is approximately twice that of seronegative herdmates.47 The absence of seroconversion (from seronegative to seropositive status in adult animals) in these herds strongly indicates maintenance of herd infection by the primary means of placental or congenital transmission.
Congenital transmission may explain the endemic pattern of abortion observed in herds suffering annual abortion rates exceeding 5% for several years.51 Epidemic patterns of abortion, however, also may be seen—that is, a high percentage of abortions may occur during a relatively short period of time.25,52 Several studies have shown a three- to fourfold increase in abortion risk for seropositive cows compared with that for seronegative herdmates.53,54 Repeat abortion in the same cow has been observed in an infected herd32 and was proved in four cows in a California dairy herd.6 Furthermore, the cow with a history of previous abortion as a result of N. caninum infection has up to a 5.7 times greater risk of abortion in the subsequent pregnancy.55 The serologic findings in California drylot dairies suggest that repeat abortions are caused by recrudescence of latent infection, although acquisition of a new infection also is possible.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree