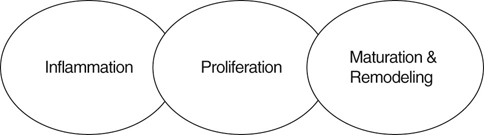
Patients undergoing rehabilitation frequently have wounds, including surgical wounds and wounds resulting from nonsurgical trauma. Therefore, an understanding of wound healing and the care of wounds is necessary to speed the rehabilitation process and identify abnormal wound healing. Normal restoration of injured tissue follows a series of predictable overlapping events often grouped as the inflammatory, proliferation, and maturation/remodeling phases of wound healing (Figure 36-1). Biochemical substances modulate cellular activities occurring during the phases of tissue repair. Acute wounds tend to follow a predictable progression through the wound healing phases, whereas chronic wounds may have a prolonged period of inflammation, proliferation, or excessive wound contraction. The first response to injury is inflammation, a process that includes vascular and cellular events to control blood loss, combat bacterial invasion, and set the stage for further healing by signaling the cells necessary for repair and regeneration to come to the site of injury. For a few seconds to several minutes following injury, blood vessels in the area reflexively constrict to reduce blood loss. Platelets aggregate at the injury site and are activated by contact between damaged endothelial cells lining the blood vessel walls and exposed collagen. The activated platelets adhere to one another and the injured endothelium and interact with fibrin to help form a plug to wall off the injured area. The activated platelets also release chemical mediators such as cytokines (signaling proteins), growth factors (hormone-like substances that control cell metabolism), and chemotactic agents (substances that attract cells important for repair to come to the site of injury).1,2 Following vasoconstriction blood vessels dilate, allowing more fluid, inflammatory cells, and growth factors into the interstitial space. The walls of injured blood vessels allow fluid to leak into the interstitial space, causing localized edema that serves to increase pressure in the interstitial space and decrease pressure in the blood vessels. As the wound area accumulates more proteins, white blood cells, and cell fragments, the fluid in the interstitial space becomes more viscous and changes from a transudate to an exudate. Mast cells in the area release inflammatory mediators and secrete enzymes. Vasodilation is induced by histamine released by mast cells and prostaglandins released by injured cells.3 Swelling and chemical release in the area continue to increase, causing pain. The entire series of events leads to the hallmark signs of inflammation: edema, heat, redness, pain, and decreased function. Cellular events that accompany the vascular events include margination of neutrophils to the sides of the blood vessel wall and eventual migration through the wall. Guidance to the injured area occurs as neutrophils follow the chemical gradient formed by bacteria and dead or dying cells through a process called chemotaxis. Circulating monocytes migrate into the tissue spaces and are then called macrophages. Neutrophils and macrophages release enzymes, engulf bacteria, produce tissue proteases that further degrade debris, and release chemotactic agents and chemical mediators that attract more inflammatory cells to the area. Although less effective than neutrophils in the phagocytic process, macrophages produce numerous growth factors and are critical in directing the tissue repair process.4,5 Fibroblasts from the local tissue and general circulation begin to fill the spaces between the capillary loops. As the fibroblasts proliferate among the capillary loops, an extracellular matrix composed of water, proteoglycans, and other cells is formed. The matrix fills the space between collagen and elastin fibers in the wounded area, allowing more fibroblasts to migrate across the wound bed. The newly formed vascularized connective tissue is called granulation tissue (Figure 36-2). Chemotactic agents and oxygen levels guide fibroblasts to migrate and proliferate, thus filling in the wound bed. Integrins, surface cell receptors, serve as chemotactic agents and adhesion molecules to assist the migration of fibroblasts within the extracellular matrix.1 As oxygen levels increase in the wounded area, less proliferative activity is stimulated. Some of the fibroblasts transform into myofibroblasts which have contractile properties and begin to pull the wound edges inward. The wound contraction decreases the size of the defect. In larger and deeper defects, more granulation tissue is needed to fill the void calling for a greater degree of wound contraction. The shape of the wound affects the speed of contraction. Linear wounds contract faster than rectangular wounds. The slowest to contract are circular wounds. Epithelial cells (primarily keratinocytes) migrate across the granulation tissue bed from the wound margins and epidermal appendages to cover the wound defect. Growth factors stimulate epithelial cell proliferation while oxygen levels and chemotaxis guide migration across the wound bed. Ease of migration is facilitated in a moist wound environment.6 After the wound has been completely resurfaced with epithelial cells, the proliferative phase is considered complete. Although the wound bed is resurfaced through the cellular and vascular events in the inflammatory and proliferative phases, the wound is not yet completely healed. The newly formed tissue must be strengthened and reorganized through collagen synthesis and lysis in the newly formed scar tissue. Through chemical events, internal stress, and external tension, the collagen fibers transform from immature type III collagen to mature type I collagen.7 Interventions of range of motion exercise and scar tissue mobilization aid the process of scar tissue remodeling eventually achieving a balance between collagen formation and degradation to produce a strong healthy scar. Most of the remodeling of the scar occurs in the first 6 to 12 months after wound closure, but can continue for up to 2 years. Even when fully remodeled, the scar tissue attains only 75% to 80% of the strength and elasticity of the original tissue.8 A variety of factors may result in failure of an acute wound to move through the inflammatory, proliferative, and maturation phases of healing in a predictable fashion. Wounds may become chronic because of a larger number of proteases and lack of protease inhibitors, leading to a degradation of the extracellular matrix and a larger wound bed. Chronic wounds have higher amounts of inflammatory immunomodulating agents and are less responsive to growth factors. Patients who are immunocompromised or who have arterial insufficiency may be unable to mount the inflammatory response necessary to clean the wound of debris and produce chemotactic cells that signal other cells necessary for the production of granulation tissue in the wounded area. Such wounds may be void of the classic signs of inflammation. In contrast, some wounds may persist in the inflammatory phase, exhibiting debris, rolled, thickened wound edges, and a lack of adherence of the wound edge to the wound bed. Common causes of chronic inflammation include the presence of a foreign body in the wound bed, repetitive trauma (e.g., excessive licking of the wound bed), and the overuse of agents that are cytotoxic to the cells involved in the healing process. Currently there are insufficient data to assess safety, efficacy, and recommendations for use of some topical agents because of inconsistency or lack of verification of equivalent comparisons between in vitro and in vivo studies.9 Agents in question include povidone-iodine, hydrogen peroxide, sodium hypochlorite bleach, and chlorhexidine.10–12 Deterrents to expedient wound healing include wound characteristics, local factors, and systemic factors (Table 36-1). Acute wounds and surgically induced wounds tend to heal more quickly than severe traumatic wounds or wounds of insidious onset. Wounds located over bony prominences, in areas with few epidermal appendages, across joints, and in areas of thick skin have slower healing times. Because more cellular activity and rebuilding of tissue are required, larger and full-thickness wounds require more healing time than smaller or more superficial wounds. The close proximity of wound edges results in shorter healing times for linear wounds than for rectangular or circularly shaped wounds. Maintenance of appropriate wound moisture within the wound bed encourages granulation tissue formation and epithelialization. Thus, wound beds and periwound areas that are too dry (desiccated) or too wet (macerated) have prolonged healing times. Devitalized tissue within the wound bed and dry exudate or scabs can slow the migration of epithelial cells across the wound bed, prolong the inflammatory response, and create an environment conducive to infection.13,14 Table 36-1 Factors Affecting Wound Healing Myers BA: Wound management: principles and practice, ed 2. Englewood Cliffs, NJ, 2008, Pearson Prentice Hall. Important to consider is the fact that all wounds are contaminated. All body surfaces, including skin, and the digestive tract have a normal microflora of bacteria and fungi that include Staphylococcus, Micrococcus, Peptococcus, Streptococcus, Acinetobacter, and yeast. The resident microflora and pH of the skin create a slightly acidic environment, serving a protective function against disease-causing microorganisms.15 In an open wound the microflora adhere to the surface and replicate, resulting in colonies that do not necessarily adversely affect the host as the normal immune response is activated. However, if the bacteria or fungi continue to replicate, a point of critical colonization is reached in which the microorganisms become a bioburden, adversely affecting the host. When microorganisms multiply to the point of invading viable body tissues, the wound is considered infected. Because of the presence of individual factors in each host, there is no specific threshold for infection, although in general, wounds with a bacterial quantity of greater than 105 microbes per gram of tissue are considered infected.16 However, the type of microorganism must also be considered because some are more virulent and produce exotoxins that may inactivate or modify host responses, leading to host cell dysfunction or death. Bacterial endotoxins, molecules within the walls of gram-negative bacteria, may induce a strong inflammatory response leading to fever, bleeding, and clotting. In addition interactions among bacteria can lead to a microorganism community called a biofilm that attaches to the wound surface and is encased in a self-developed glycocalyx (which protects the bacteria). The microorganisms encased in the biofilm exhibit increased virulence and resistance, making them less responsive to biological and pharmaceutical intervention.17 Immunocompromise, malnutrition, and impaired local circulation of the host can also affect resistance to invading organisms. In general, infection should be suspected when the signs of inflammation are disproportionate to the size and extent of the wound (e.g., drainage is copious, has uncharacteristic color, wound is very painful, periwound area is extremely red or hot, etc.). Wound cultures such as tissue biopsies, swab cultures, or fluid aspirations may be used to identify an offending microorganism. Although tissue biopsy is the gold standard for culturing, properly performed swab cultures are a reliable method to identify and quantify bacteria in a wound.18 To properly perform a swab culture, the clinician must first debride and thoroughly rinse the wound bed. The swab should then be moved slowly over viable tissue in the wound bed in a crisscross pattern back and forth, moving from top to bottom while rolling the swab and applying enough pressure to express some of the wound bed fluid. Wounds with a small surface area of little granulation tissue should be cultured by swabbing only the small area for 5 seconds while rotating the applicator.19 Tissue biopsies are preferred for evaluating pressure/decubital ulcers, burns, or wounds with a suspected neoplastic process. Medications to relieve pain should not be withheld from a patient, because analgesic agents do not prevent a full patient evaluation from being completed. Pain medications do not mask the physiologic indicators, because the heart rate will still be elevated in response to hypovolemia, hypoxia, hypotension, or hypercarbia, even when high doses of opioid medications have been delivered to the patient. In the emergency patient opioids are unlikely to cause respiratory depression or the deterioration of hemodynamic parameters.20 The use of nonsteroidal antiinflammatory drugs (NSAIDs) and corticosteroids are contraindicated for use in a patient in shock until renal perfusion, blood pressure, and dehydration issues have been addressed. In addition, endogenous endorphins released in a stressful situation, such as an emergency room, can mask the seriousness of the patient’s condition; therefore chemical sedation for fear and aggression is a humane way to examine the emergency patient. An oxygen mask or oxygen induction chamber allows the patient to relax while medications are administered. 21 Severity of the injury determines the proper medications for pain control. Severe to excruciating pain present after trauma with fractures and muscle damage require high-dose opioids and additional pain medications for adequate relief. Fractures should be immobilized as soon as possible to reduce pain. Muscle hemorrhage, hematomas, or bruising may cause significant discomfort. These injuries may benefit from local application of ice during the first 72 hours or until the acute inflammatory phase has ended, and heat application thereafter. For injuries to the perineum, pelvis, and rear limbs, epidural anesthesia may be necessary if opioids are inadequate to control pain. After the patient has been stabilized, adjunctive medications such as NSAIDs, medications for nerve pain, and low-dose sedatives may be administered.1 Although external bleeding wounds often capture the attention of the owners, the health care worker must be aware that more life-threatening injuries may be present. The initial stabilization includes an examination of the airway with administration of oxygen, as appropriate. In the case of severe head wounds, facial trauma or tracheal damage, rapid induction may be necessary to accomplish intubation and obtain an open airway (Figures 36-3 and 36-4). Puncture wounds or trauma to the chest wall should increase suspicion of a pneumothorax, for which a thoracocentesis or chest tube may be necessary. All vital signs should be obtained to evaluate the need for IV fluids and/or blood products to treat hypovolemic shock.
Physical Rehabilitation for Wound Care

Wound Healing Physiology
Inflammatory Phase
Proliferative Phase
Maturation/Remodeling Phase
Abnormal Wound Healing
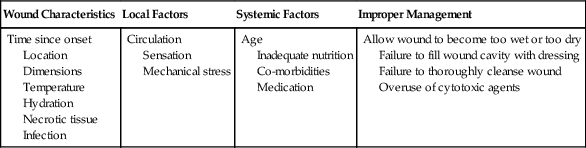
Factors Affecting Wound Healing
Wound Characteristics
Local Factors
Systemic Factors
Improper Management
Time since onset
Location
Dimensions
Temperature
Hydration
Necrotic tissue
Infection
Circulation
Sensation
Mechanical stress
Age
Inadequate nutrition
Co-morbidities
Medication
Allow wound to become too wet or too dry
Failure to fill wound cavity with dressing
Failure to thoroughly cleanse wound
Overuse of cytotoxic agents
Emergency Care
Initial Assessment
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Physical Rehabilitation for Wound Care
Only gold members can continue reading. Log In or Register to continue