Chapter 28 Peritoneal Dialysis
Biology of the peritoneal membrane
The peritoneum is the serosal membrane that lines the abdominal cavity. The portion that covers the viscera and other intraabdominal structures is known as the visceral peritoneum, and that which lines the abdominal cavity is known as the parietal peritoneum. In humans, the surface area of the peritoneum is approximately the same as the body surface area (1 to 2 m2), and the visceral peritoneum accounts for approximately 80% of the total.10 Peritoneal surface area is proportionately larger in comparison to body surface area in infants and children,11 suggesting that this difference would also be true for dogs and cats.
Anatomically, the peritoneum consists of the mesothelium and underlying interstitial tissue (Figure 28-1). The mesothelium consists of a simple squamous epithelial-like monolayer supported by a basement membrane. The mesothelial cells have many apical microvilli that increase the functional surface area of the membrane. In humans, the basement membrane contains type IV collagen, proteoglycans, and glycoproteins. The interstitium is a layer of connective tissue below the basement membrane. Found within the connective tissue are extracellular matrix molecules, including collagen, fibronectin, and elastin. This layer has a gel-like character because of the presence of various proteoglycans. The peritoneal microvasculature is composed of true capillaries and postcapillary venules, which are supported by a negatively charged glycocalyx.61 These vessels are located at various distances from the mesothelial surface and can be found throughout the connective tissue layer. Lymphatics also are found in this layer, most commonly in the subdiaphragmatic peritoneum. These lymphatics drain primarily via stomata in the diaphragmatic peritoneum.10,48 The role of lymphatics in fluid and solute exchange from the peritoneum is poorly understood because of the difficulty in directly measuring lymph flow. Lymph flow is affected more by gravity than is blood flow through vessels, and therefore the upright posture of humans versus the quadruped stance of animals may mean that the role of peritoneal lymphatics differs between species.
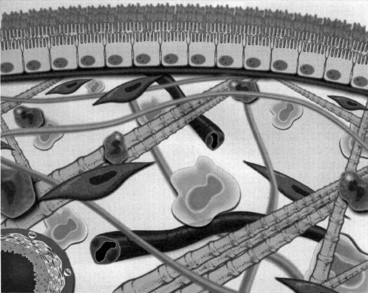
Figure 28-1 Diagrammatic representation of the peritoneal membrane.
(From Nagy JA, Jackman RW. Dialysis and transplantation: a companion to Brenner & Rector’s the kidney. Philadelphia: WB Saunders, 2000: 110.)
The most important function of the peritoneal membrane is to provide a protective, lubricating surface for the abdominal organs. Mesothelial cells secrete glycosaminoglycans including hyaluronic acid, proteoglycans such as decorin and biglycan, and phosphatidylcholine-containing lamellar bodies that allow free movement of the visceral organs during respiration and gastrointestinal peristalsis.48,61 Mesothelial cells play a role in a number of other processes, including antigen presentation, control of inflammation, tissue repair, coagulation, and fibrinolysis.13,35 It is generally believed, however, that the mesothelium does not represent a significant barrier to water transport.61 The anatomic structures that appear to play the most important role in fluid and solute transport are the walls of the capillaries and the extracellular matrix located in the submesothelial cell connective tissue.27,28,63 Peritoneal capillaries are composed primarily of nonfenestrated endothelial cells supported by a basement membrane. Endothelial cells contain aquaporins, which are 20-kDa cellular membrane proteins that are responsible for water transport. Intercellular clefts between endothelial cells also play a role in solute transport.36
Although the anatomic surface area of the peritoneum is large, the effective surface area—that area involved in fluid and solute movement—is considerably smaller. This discordance is because transport of water and solutes is primarily dependent on the surface area of peritoneal capillaries, rather than the mesothelium.3,10,36
Fluid and solute transport
The mechanisms by which fluids and solutes are transported across the peritoneal membrane involve several physical processes, including diffusion, convection, and ultrafiltration. Diffusion can be defined as the tendency for solutes to disperse within the available space.11 Solutes move by osmosis from a space with a higher concentration of that solute to one with a lower concentration. When this movement occurs across a semipermeable membrane, the rate of diffusion is governed by the permeability of the membrane, the available surface area for diffusion, and the concentration of solute on either side of the membrane. Diffusion is most rapid when the two solutions have markedly different solute concentrations, and the rate of movement of solute slows as the concentrations become more equal. Diffusion continues until the solutions on either side of the semipermeable membrane are of equal solute concentration.
Ultrafiltration is the removal of fluid (water) during PD. The rate of ultrafiltration is dependent on the osmotic or oncotic gradient between peritoneal capillary plasma and dialysate, as well as the effective peritoneal surface area and capillary blood flow.61,68 In PD, ultrafiltration is accomplished by instilling fluid into the peritoneal cavity that is of higher osmolality than that of plasma. Ultrafiltration is frequently desired when performing PD because animals are often overhydrated as the result of fluid administration.
Convection occurs when solutes are carried along with the bulk flow of water during ultrafiltration. This movement can occur even when the concentrations of solute on either side of the semipermeable membrane would not promote diffusion of the solute. This effect does not play an important role in PD; it is more important in hemodialysis, where this process can be mechanically manipulated (see Chapter 29).
Transport of water and solutes across the peritoneal membrane is best explained by the three pore theory.* Large pores, 100 to 200 Å in diameter, correspond to clefts in the endothelium and allow the transport of macromolecules such as albumin. They are present in very small numbers, accounting for less than 0.01% of the total pore surface area. Small pores, 20 to 25 Å in diameter, also correspond to clefts in the endothelium. They present in large numbers, representing more than 90% of the pore surface area, and allow the passage of low molecular weight substances such as urea. creatinine, and glucose. Ultrasmall pores, 4 to 6 Å in diameter, are aquaporin I channels found within peritoneal capillary and mesothelial cells, and transport only water.21,40,50–52,59,61 Aquaporins are a family of transmembrane polypeptides that permit water transport across the cellular membrane in response to an osmotic gradient.21,51,59,71 Aquaporin expression in mesothelial cells can be induced by exposure of the cells to hyperosmotic solutions.50
It has also been postulated that the location and the number of peritoneal capillaries affect the rate of transport of fluid and solutes.10,41,56 Those capillaries that are located farther from the mesothelium would participate less in the transport process.
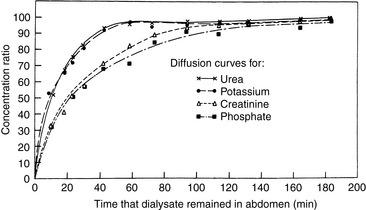
In PD, diffusion is responsible for the transfer of urea, creatinine, and other small solutes from the compartment in which they are present in high concentration (plasma in peritoneal capillaries) to that in which they have low or no concentration (dialysate). The other factor affecting diffusion is the ability of a membrane to transport the specific solute that is directly proportional to the effective peritoneal surface area, and inversely proportional to the overall resistance. The mass transfer area coefficient (MTAC) is the theoretical clearance rate that would occur if the concentration gradient for a solute is infinitely high. The osmotic gradient, MTAC, and rate of diffusion are highest at the beginning of a dwell cycle, when the concentration gradient is highest.61,68 The rate of removal of a substance by diffusion is not only related to osmotic gradients but also to the size of the molecule and to the area available for diffusion. Urea has a relatively low molecular weight of 60 and diffuses more rapidly than creatinine, which has a molecular weight of 113. Larger molecules such as albumin (MW 69,000) are dependent on diffusion through larger pores, and the rate is comparably slower (Figure 28-2).
Ultrafiltration is the removal of fluid (water) during PD. The rate of ultrafiltration is dependent on the osmotic or oncotic gradient between peritoneal capillary plasma and dialysate, as well as the effective peritoneal surface area and capillary blood flow.61,68
Convection (solvent drag) is the movement of solutes accompanying the flow of water from peritoneal capillaries into the peritoneal cavity. For most solutes, movement by convection does not occur in direct proportion to their concentration in blood. This effect is termed sieving and occurs because there is a greater barrier to solute than water movement across the peritoneum. Sieving coefficients vary depending upon the charge and molecular weight.10,15,36,61,68 As a result of sieving, the rate of decrease in solute concentration gradient gradually slows with longer dwell times. In people, physical properties of the peritoneal membrane vary, resulting in different coefficients. People treated with chronic PD undergo testing to determine the rate of ultrafiltration and solute clearance. One such test measures the rate at which creatinine appears in the dialysate compared with its concentration in plasma. The reason for performing such tests is that humans who are treated with chronic peritoneal dialysis have or develop changes in the peritoneal membrane that affect the rate at which solutes are transported. In low solute transporters, the osmotic gradient between plasma and dialysate remains high for a longer period, and therefore there is a high rate of ultrafiltration of water into dialysate. In high transporters, there is more efficient removal of urea, creatinine, and other uremic substances, but ultrafiltration is less efficient. In average transporters, the rates of solute and water movement are intermediate between those of low and high transporters.11,36,49 There is no such corresponding information available for clinical use in dogs and cats. Although such information would be useful in formulating an accurate dialysis prescription, its benefit in the treatment of acute disease is likely less important than for chronic disease.
Changes in the peritoneal membrane in chronic dialysis
Peritoneal dialysis is one of the methods of chronic renal replacement therapy in humans. Repeated, long-term instillation of dialysate fluid into the peritoneal cavity (3 to 4 years) results in a number of pathophysiologic changes that result in decreased diffusion and convection across the peritoneal membrane. Glucose-degradation products (GDPs) in PD fluids are most commonly implicated in membrane failure.61 Because virtually all of the PD done in veterinary patients is short-term, these changes are not likely to affect clinical management.
Indications for peritoneal dialysis
The primary indication for PD in animals is for the treatment of acute kidney injury. This includes oliguric or anuric renal failure, acute polyuric renal failure with severe uremia that is unresponsive to fluid therapy, and postrenal uremia resulting from ureteral obstruction or a rupture in the urinary collecting system. Although PD is less efficient than hemodialysis in correcting uremia, and water and solute abnormalities, it still has a number of therapeutic advantages (Box 28-1). The decreased efficacy may be beneficial in treating cats and small dogs, in which rapid water and electrolyte shifts can result in serious clinical consequences. The equipment and supplies used for PD are easily obtained, and the technique for performing PD, although labor intensive, is not difficult. This makes PD a useful therapeutic modality for private practices, especially those located in areas distant from hemodialysis facilities.
Although acute kidney injury is the most common indication for PD, it is not the only one. PD can be used for treatment of toxicities in which the offending toxin can be removed by diffusion across the peritoneal membrane. Such toxins include ethylene glycol, ethanol, and barbiturates. Severe metabolic disturbances, such as hypercalcemia, hyperkalemia, hepatic encephalopathy, and resistant metabolic acidosis, also can be corrected with PD. PD with hypertonic dialysate can be used to remove excess body water in animals with life-threatening fluid overload, such as may occur with heart failure. There are other disorders in which peritoneal lavage, using solutions and techniques very similar to those for PD, may be beneficial. These include hypothermia, hyperthermia resulting from heat stroke, and pancreatitis (Box 28-2).23
Box 28-2 Indications for Acute Peritoneal Dialysis
Published reports of PD in dogs and cats have noted varying outcomes.* Although most described improvement in renal function during dialysis, overall survival remained poor. More recent publications reported improved survival, perhaps indicating an improvement in technique or overall management of critically ill animals. In one study of 27 dogs and cats, 24% improved and were discharged from the hospital.20 However, 11 of 21 of the animals with acute renal failure suffered from ethylene glycol intoxication, which is known to result in a mortality rate approaching 100%.30,69 In another study, 45% (10 of 22) of cats treated with PD were discharged from the hospital.16 One study of PD for the treatment of leptospirosis in five dogs reported a survival rate of 80%.9 Complications identified in these dogs were hypokalemia (60%), hypoalbuminemia (40%), hypomagnesemia (20%), pelvic limb edema (40%), central nervous system signs (40%), dialysate retention (20%), and leakage from the catheter site (20%). A report of six cats treated with PD for acute renal failure of varying causes reported survival in five of the six cats, all of which eventually had normal renal parameters. All cats had complications, including subcutaneous edema (83%), hyperglycemia (67%), dialysate retention (50%), and hypoalbuminemia (50%).22
The success rate of PD must be compared with the overall survival rate of animals with acute renal failure treated with other means because animals undergoing dialysis traditionally have been those with the most severe renal failure. The survival rate of dogs with leptospirosis treated with fluids and antibiotics has been reported to be 59% to 85%.1,33,54 In a study of 99 dogs with acute renal failure, 43% were discharged from the hospital. Of these, 24% were left with residual renal dysfunction, and only 19% had return to normal renal function.69 In another report of 80 dogs with acute renal failure, only 20% survived to discharge; 44% of the dogs had ethylene glycol intoxication.30 It may be that the survival of animals with acute kidney injury treated with PD is more dependent on the underlying cause of disease than the technique.
PD could theoretically be used for the long-term management of animals with chronic renal failure. However, technical problems with catheter flow and complications such as infection make chronic PD difficult. Few such cases have been reported.12,20,58,62,67
Contraindications to peritoneal dialysis
There are few situations in which PD is absolutely contraindicated. In humans, these include peritoneal adhesions that prevent fluid distribution throughout the abdominal cavity and pleuroperitoneal leaks that would result in pleural effusion and respiratory compromise. Although adhesions are common in humans, especially those that have had abdominal surgery,13 they are not often seen in dogs and cats. Diaphragmatic or pericardiodiaphragmatic hernias are seen in animals and could result in respiratory or cardiac dysfunction. That said, pleural dialysis has been described in two dogs with acute renal failure.60 Relative contraindications for PD include recent thoracic or abdominal surgery, inguinal or abdominal hernias, and severe hypercatabolic states, such as those seen with cutaneous burns or skin denudation. Animals with recent abdominal surgery, especially gastrointestinal surgery, are at risk for dehiscence and infection during PD because of the increased abdominal pressure and potential fluid leakage through the incision site. Similarly, progressive herniation may occur as the result of increased intraabdominal pressure. Concomitant catabolic diseases contribute to the hypoalbuminemia that can occur during PD.
Protocol for peritoneal dialysis
Catheter types and placement
The key to successful PD is the catheter and its placement. An ideal catheter allows efficient inflow and outflow, is biocompatible, resists infection of both the peritoneum and subcutaneous tunnel, and retards leakage at the peritoneal exit site.18 One of the most common causes of catheter failure in small animals is catheter obstruction by omentum, resulting in failure to drain dialysate from the abdomen. There are many catheter designs available (Box 28-3), and most are modifications of a fenestrated silicone tube with Dacron (Invista, Wichita, Kan.) cuffs applied to promote fibrous attachments at the peritoneal and cutaneous exit sites (Figure 28-3). Simple tube catheters with stylets can be placed percutaneously in conscious animals using local anesthetics in an emergency situation.23,24,53 A percutaneous cystotomy tube catheter (Stamey percutaneous suprapubic catheter set, Cook, Spencer, Ind.) also has been used successfully for acute short-term PD (Figure 28-4). The Tenckhoff catheter, developed in 1968, is a straight soft Silastic tube fenestrated at the distal end and furnished with two Dacron velour cuffs.65 Because of the high rate of omental entrapment, surgical omentectomies are advocated when using this catheter in animals in which more than 3 days of dialysis is anticipated.12,23,24 PD for acute renal failure should be performed for a minimum of 48 to 72 hours, but the animal’s condition often necessitates a longer period of treatment.
Box 28-3 Suppliers of Peritoneal Dialysis Catheters
Ash Advantage Peritoneal Catheter
Coiled Adult Standard and Large
Kendall Healthcare/Kendall Vascular
A variety of peritoneal dialysis catheters
Straight and curled, one and two cuff peritoneal dialysis catheters
Acute and chronic peritoneal dialysis catheters, Tenckhoff and Spiral
Global Veterinary Products, Inc.
Acute peritoneal dialysis catheter, coaxial design
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree