Chapter 26
Peripheral Blood Smears
Blood smear preparation is easy and inexpensive, and smear examination is readily learned with adequate background information and routine practice. This chapter describes techniques of preparation and interpretation of canine and feline blood smears and addresses integrating findings from blood smears with other parameters in CBCs. Supplementary information on interpreting routine hematologic parameter values is available in several well-written reviews and texts.1–5
Sample Collection
Alternatively (particularly if sample volume is limited), blood without an anticoagulant may be placed directly from the collection needle onto the slide. Samples collected from superficial skin-puncture wounds or clipped toenails (because of excessive contamination with tissue procoagulants) and blood anticoagulated with heparin (a relatively poor preservative of cellular morphology and staining characteristics) are less acceptable. Blood anticoagulated with citrate may be used to evaluate cell morphology on blood smears and may be particularly useful to avoid anticoagulant-associated pseudothrombocytopenia; however, the required 10% sample dilution interferes with cell count estimates.
Hematologic Reference Intervals
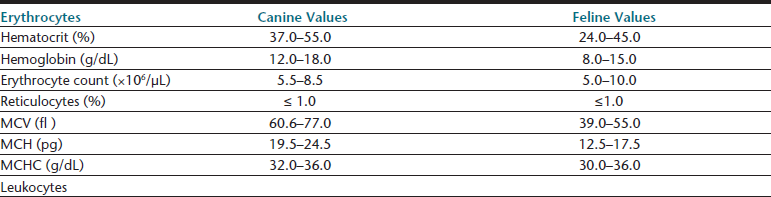
Hematologic reference intervals should be established by individual diagnostic laboratories; however, published reference ranges may be used as a general guide for in-clinic laboratories, though most instruments will come with some reference interval information generated on that methodology. Typical values for dogs and cats are listed in Table 26-1. Certain physiologic factors occasionally cause a healthy patient’s hematologic values to deviate from reference ranges. Because they are rapidly expanding their vascular space, very young animals tend to have relatively low hematocrits. Because they are also actively replacing fetal with adult RBCs, animals in early growth periods have greater RBC anisocytosis, polychromasia, and incidence of nucleated RBCs compared with mature animals.
Relatively high lymphocyte counts are also common in young animals, and lymphopenia is suggested if lymphocyte counts drop below 2000 cells per microliter (cells/µL) in puppies and kittens under 6 months of age.3 Transient elevations above the reference range for lymphocyte counts and occasionally reticulocyte counts may be seen in excited or vigorously exercised patients, especially if they are immature. This epinephrine-induced response may result in temporarily increased counts of other WBC types in the peripheral blood of healthy patients. At least one canine breed, the Greyhound, has hematologic reference ranges reported to fall slightly outside of reference ranges commonly used for the species.6–8 Greyhound crossbreed dogs may also have similar changes.9 Although these normal physiologic conditions should be considered, they generally explain less than 5% of the patient values that fall outside reference ranges for any single hematologic parameter.
Smear Preparation
Glass Slides
Smears are prepared on glass slides by placing a drop of blood (2 to 3 millimeters [mm] in diameter) on the broad face of the slide about 1.0 to 1.5 centimeter (cm) from the frosted border (or edge of a nonfrosted slide). Another clean, dry slide (i.e., spreader slide) is held loosely against the surface of the first slide at a 30-degree angle and drawn smoothly toward the blood drop, as illustrated in Figure 1-17. The spreader slide should be brought to a position where it just meets, but is not drawn into, the blood drop. When the spreader slide makes contact with the blood, capillary action immediately distributes the blood between the two slides. Then, with no downward pressure, the spreader slide is quickly and smoothly swept across the remaining length of the underlying slide.
Ideally, blood smears have a smooth transition from the thick region to the feathered edge and cover an area half the length and slightly less than the width of the slide (see Figure 1-17, D). If the edge is blunt instead of feathered, the second slide was probably raised off the first before the blood was spread completely. Unequal smear thickness usually results from the spreader slide being held at too obtuse an angle or placing too much pressure on the first slide while spreading the blood. Too much pressure on the second slide may also result in WBCs clumping along the smear’s feathered edge. Too little pressure may result in short, thick smears. Smear thickness may also be affected by the viscosity of the blood sample. Adjusting the angle at which the second slide is held against the first may help compensate for very viscous (e.g., hemoconcentrated) or watery (i.e., anemic) blood samples. A more obtuse, 40- to 45-degree angle between the two slides makes thicker smears for very anemic samples, and an angle less than 30 degrees may be necessary for preparing smears of severely hemoconcentrated blood. Smears need to be thoroughly air-dried before staining; those that are thick may require additional drying time. Use (at the low setting) of a heat block or a blow dryer may shorten the drying time.
Stains
Quick stain methods vary somewhat and should be used according to manufacturer’s recommendations. The two-step Prodiff is unique among quick stains in differentially staining polychromatophilic and mature RBCs. Most quick stains such as Diff-Quik, Hemacolor, and Quick III lack good distinction of polychromatophilic erythrocytes but provide particularly consistent staining between smear preparations. The quick stains generally require slowly dipping the smear first in an alcohol fixative, then in a methylene blue dye mixture, and lastly, in an eosin-containing solution. One edge of the slide is briefly blotted between solutions. Tap or distilled water may be used to rinse the slide after the last solution and before air-drying.
Troubleshooting
Artifacts of Cell Morphology and Staining
Crenated Erythrocytes
Crenated erythrocytes (see Figure 26-36), artifacts especially common in feline blood samples, have a thorn-apple shape with many short, uniformly spaced, blunt or pointed spicules that protrude from the cell membrane. Crenated RBCs may result from drying the smear too slowly or a relative excess of anticoagulant in the sample. Prolonged storage time of the blood (e.g., more than 2 hours at either 4°C or room temperature), particularly with an EDTA anticoagulant, may also result in RBC crenation. Differences in surface tension between the cell membrane and the glass slide may be an unpredictable cause of the artifact.
In Vitro Aging Artifacts
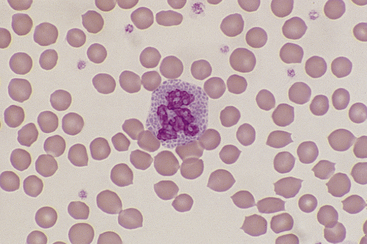
These are common in smears made from blood samples left at room temperature for more than 2 to 4 hours or refrigerated for longer than 12 hours. Neutrophils, monocytes, and immature and neoplastic WBCs degenerate slightly earlier than other cell types. The nuclei of affected cells initially become condensed and stain homogeneously and segmentation of granulocyte nuclei becomes more prominent, with only thin strands of chromatin separating lobules (e.g., hypersegmented neutrophils). Basophilia and vacuolation may be evident in the cytoplasm of neutrophils, and nuclei later become pyknotic and fragmented. The cytoplasmic borders of degenerating cells often show blebbing (Figure 26-1), and the cytoplasm of lymphocytes and monocytes may become vacuolated. These changes interfere with accurate identification of cell type and invalidate differential WBC counts if more than 10% of the cells in the smear are affected. Platelet aggregation or clumping is also prevalent in aged samples. Avoiding aging artifacts is one reason to include fresh well-made smears with a sample submitted for hematology analysis to an outside laboratory.
Pale or Unstained Nuclei
Pale or unstained nuclei (Figure 26-2) on smears suggest that inadequate staining time has been allowed or that the stain has aged. Of the three-step quick stain solutions, it is the methylene blue mixture that has begun to degrade and may require longer incubation time with the smear or replacement. On Wright-stained preparations, pale nuclei with bright orange-red RBCs may result from overzealous washing or low pH of the buffer. Conversely, Wright-stained smears with pale nuclei and RBCs that stain slightly brown to green may occur when preparations are thick, inadequately washed, or stained with too little or too alkaline a buffer. A neutral buffer (i.e., pH 6.4 to 7.0) is most effective for Wright staining. The pH of the distilled or tap water wash solution may occasionally interfere with the intensity of the nuclear staining with both Wright and quick stains. Additional causes of inadequate nuclear staining are noted in Table 1-3.
Drying Artifact
Drying artifact (Figure 26-3 and Figure 26-4) results if a smear is insufficiently air-dried before it is stained. The artifact is recognized in RBCs as round to crescent-shaped, punched-out regions or refractile vacuole-like structures. Stain condensations may border these pale cell areas or precipitate in the background between cells.
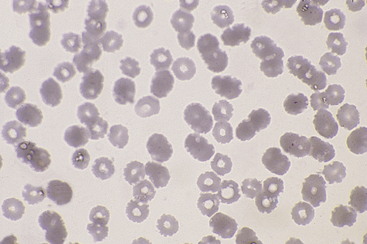
Figure 26-3 This blood film was inadequately dried before staining. The punched out, unstained regions in the red blood cells are a result of moisture interfering with the contact between cells and stain. (Diff-Quik stain. Original magnification 250×.)
Overall Blue Tint
This may be seen on blood smears stained after prolonged storage, prepared from heparin-anticoagulated blood, or exposed to formalin or to formalin fumes before staining. Even indirect exposure to formalin fumes (as may occur when slides are packaged with fixed tissues for shipment) may result in an overall pale, hazy, and often basophilic smear (Figure 26-5).
Stain Precipitate
Stain precipitate is more often a problem with Wright stains than with quick stains. Wright stain will precipitate in storage, if incubated on a slide too long, or if insufficiently washed from a slide after incubation. Precipitate formed during storage and from insufficient washing occurs as random aggregates of spherical and dumbbell-shaped granules that appear both in and out of the smear’s plane of focus (Figure 26-6). With prolonged incubation time, stain precipitate appears throughout the smear as uniformly dispersed, irregular globules of stain. Precipitate formed during storage may be removed by filtering the stain through Whatman filter paper into clean vials.
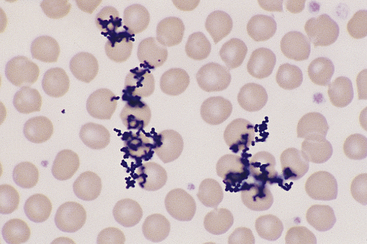
Figure 26-6 The granular stain precipitate appears in and out of the plane of focus for the smear. The precipitate formed as a result of insufficient washing of Wright stain after buffer application. The granular precipitate that forms in the stain during storage may also be deposited on blood smears. The latter may be prevented by filtering the stain before use. (Wright stain. Original magnification 330×.)
Blood Smear Evaluation
A rough approximation of the patient’s hematocrit may be obtained by examining a blood smear at low magnification. Blood films from nonanemic animals generally have RBCs that are closely apposed in the monolayer as well as several RBC layers at the thick end of the smear that obstruct penetrance of most condenser light. In contrast, smears from animals that are moderately to markedly anemic usually have RBCs that are widely separated from one another in the monolayer and only one or two RBC layers in the thick end of the smear that allow considerable condenser light to penetrate. Unless the angle between slides was adjusted during smear preparation of hemoconcentrated samples, the monolayer occupies a relatively reduced area. Estimates should ultimately be checked against the patient’s measured hematocrit or packed-cell volume.
Normal Cell Components of Blood
White Blood Cells
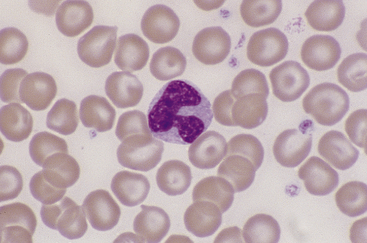
Neutrophils
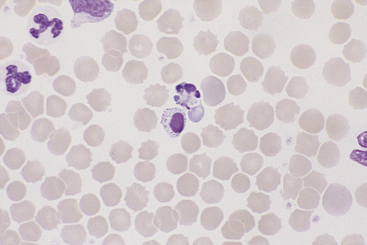
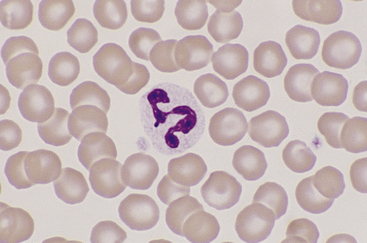
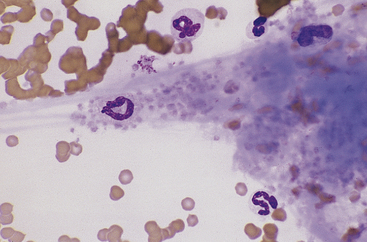
Canine and feline neutrophils have similar appearance on blood films (Figure 26-7). The neutrophil nucleus is elongate and separated into multiple lobules by invaginations of the nuclear border. Demarcations between lobules are seldom distinct enough to be considered filamentous. Chromatin is organized into dense clumps of dark purple to black staining heterochromatin separated by narrow areas of less condensed euchromatin. Cytoplasm is clear, pale eosinophilic to faintly basophilic with a fine grainy texture and, rarely, contains one or two small vacuoles. Neutrophil granules range from indiscernible to faintly eosinophilic but are pale and much smaller than the prominent granules of mature eosinophils.
Band Neutrophils
Band neutrophils, low numbers of which occur in the peripheral blood of healthy dogs and cats, have an elongate, U- or J-shaped to slightly twisted nucleus with less chromatin condensation compared with mature neutrophils (Figure 26-8). Nuclear lobulation is absent or poorly defined. Constrictions of canine band neutrophil nuclei are less than half the width of the remainder (nonconstricted sections) of the nucleus; feline band neutrophils lack nuclear constrictions entirely. Cytoplasm is similar in granule content and staining to that of mature neutrophils.
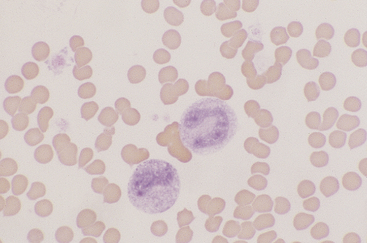
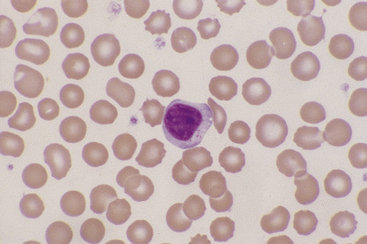
Monocytes
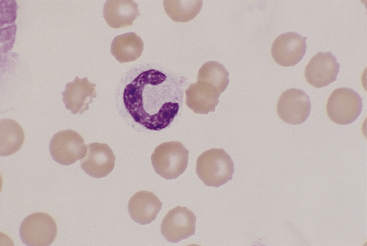
Canine and feline monocytes are larger than neutrophils and similar in size to eosinophils and basophils. Nuclei vary greatly in morphology, ranging from elongate “U” shapes, which resemble band neutrophils to irregular multilobulated forms. The nuclear chromatin of monocytes is generally distinct from that of both mature and immature granulocytes and is characteristically lacy to ropy with only a few small isolated clumps of heterochromatin (Figure 26-9). The moderate to abundant gray-blue cytoplasm of monocytes has a ground-glass texture, is often sparsely dusted with minute eosinophilic granules, and occasionally contains vacuoles. Cytoplasmic borders are usually irregular, sometimes with fine, filamentous, pseudopodia-like extensions. Because of their relatively large size, monocytes may be concentrated along the feathered edge, and their proportion underestimated in blood smear differential WBC counts.
Lymphocytes
Lymphocytes vary in size in the peripheral blood of dogs and cats, with small cells predominating. Small lymphocytes have densely staining, round to oval nuclei that are sometimes slightly indented and usually have large, well-defined chromatin clumps (Figure 26-10). Alternatively, nuclear chromatin may appear smudged, especially when stained with a quick stain. The moderately blue cytoplasm of small lymphocytes is scant, and cytoplasmic borders are partially obscured by the nuclei, particularly with feline lymphocytes. Larger lymphocytes in peripheral blood have less densely staining, but still clearly clumped, nuclear chromatin. Cytoplasm of the larger cells is more abundant and ranges from light to moderately basophilic. Some lymphocytes have a few variably sized eosinophilic cytoplasmic granules that are usually concentrated within a single perinuclear cell area (Figure 26-11).
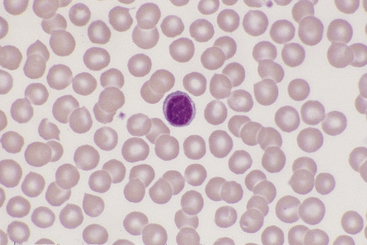
Figure 26-10 A canine lymphocyte with densely clumped nuclear chromatin and scant basophilic cytoplasm. (Wright stain. Original magnification 330×.)
Eosinophils
Eosinophils, which are slightly larger than neutrophils, may usually be found in very low numbers on blood smears of healthy dogs and cats. Nuclei are less lobulated (often being divided into only two distinct lobules) with less condensed chromatin (Figure 26-12) than those of mature neutrophils. Cytoplasm is clear to faintly basophilic and contains prominent pink granules, which are abundant, small, and rod-shaped in cats (Figure 26-13) but vary widely in number and size in dogs. Canine eosinophils occasionally contain a single, large granule that may be mistaken for an inclusion body or unusual organism (Figure 26-14). Eosinophils of Greyhounds are peculiar in that they may appear vacuolated on smears—a breed difference that has been attributed to differential staining properties of the specific granules (Figure 26-15).10 Eosinophil granules that are ruptured in vitro are also sometimes freely scattered in the background of canine and feline blood smears.
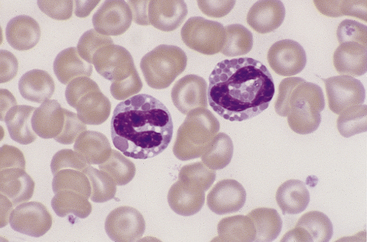
Figure 26-12 Two canine eosinophils, one of which has partially degranulated cytoplasm. Eosinophil nuclei are less condensed and lobulated than those of neutrophils. (Wright stain. Original magnification 330×.)
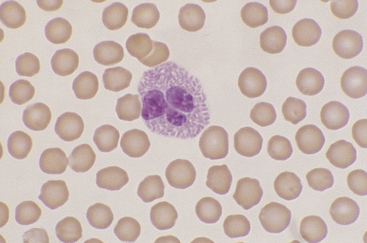
Figure 26-13 A feline eosinophil with small, rod-shaped, eosinophilic granules filling the cytoplasm and partially obscuring the nucleus. (Wright stain. Original magnification 330×.)
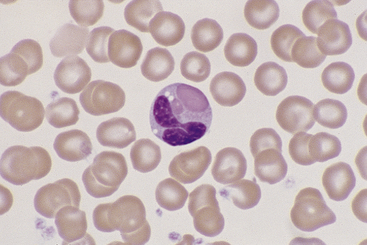
Figure 26-14 A canine eosinophil with only two large cytoplasmic granules. (Wright stain. Original magnification 330×.)
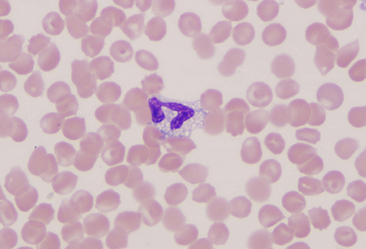
Basophils
Basophils are the largest of the mature granulocytic cell types and rare in peripheral blood of healthy dogs and cats. Nuclei are less densely staining and have fewer lobulations and a more elongated, ribbon-like appearance than the nuclei of other granulocytic cell types (Figure 26-16). Cytoplasm is moderately blue-gray to slightly purple and usually contains granules. In dogs, basophil granules are usually low in number and stain dark blue to metachromatic. Canine basophils also occasionally lack obvious granules but are recognizable by their size, nuclear morphology, and cytoplasmic staining (Figure 26-17). In cats, basophils contain abundant oval, pale lavender to gray specific granules (Figure 26-18), although immature basophils may also contain a few primary, dark purple granules.
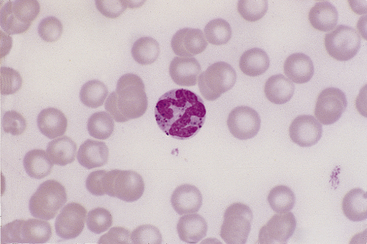
Figure 26-16 A canine basophil with metachromatic granules scattered in the cytoplasm. (Wright stain. Original magnification 250×.)
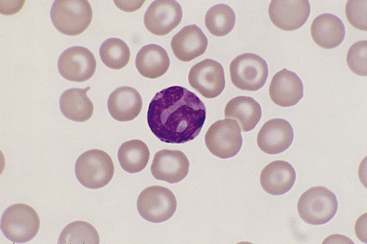
Figure 26-17 A poorly granulated canine basophil, which may be identified as such by its size, ribbonlike nuclear shape, and cytoplasmic staining, despite the near absence of cytoplasmic granules. (Wright stain. Original magnification 330×.)
Platelets
Canine and feline platelets appear oval, round, or rod shaped on peripheral blood smears. Their clear to pale gray cytoplasm usually contains a central cluster of eosinophilic to metachromatic granules. Platelets normally vary in size from about one fourth to two thirds of the diameter of RBCs in canine blood, and occasionally are even larger than the RBCs in feline blood. These larger platelets may be noted as macroplatelets in hematology reports. Partially activated platelets have a spider-like appearance with thin cytoplasmic processes extending from a small spherical cell body. Platelets may also appear aggregated (Figure 26-19) or clumped into an amorphous mass on blood smears, a finding particularly common in feline blood smears. Pale blue fibrin strands indicating active clotting and fibrin formation may also be noted. Aggregated and clumped platelets are usually pushed to the feathered edge, which may result in the false impression of thrombocytopenia if only the monolayer of the smear is evaluated.
Alterations of Rbcs in Disease
Alterations in Red Blood Cell Numbers
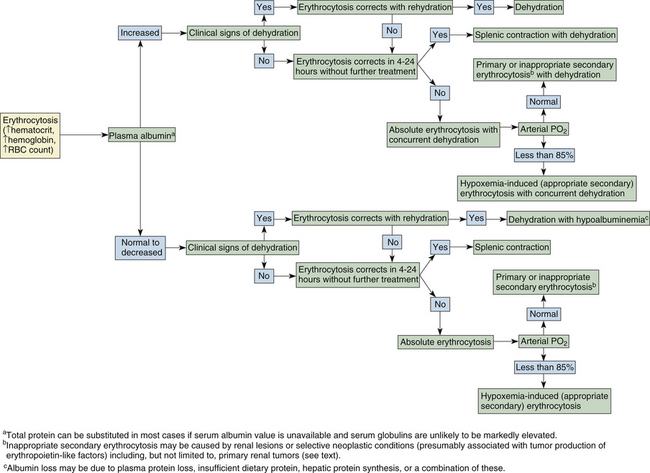
Absolute erythrocytosis secondary to inappropriate erythropoietin production, and primary polycythemia, or polycythemia vera, which is independent of erythropoietin levels, are both rare in dogs and cats. These two conditions are tentatively diagnosed by excluding the more common causes of erythrocytosis. Erythrocytosis associated with tumor erythropoietin production has been reported in dogs with renal and nonrenal tumor types, with the latter including cecal leiomyoma, nasal fibrosarcoma, and extradural schwannoma.11–13 Inappropriate administration of recombinant erythropoietin or androgens may also be a cause of absolute secondary erythrocytosis in dogs or cats as in other species, although treatment with human erythropoietin may also lead to red cell aplasia in both species.14 The algorithm of Figure 26-20 may further help in determining the cause of erythrocytosis in dogs and cats.
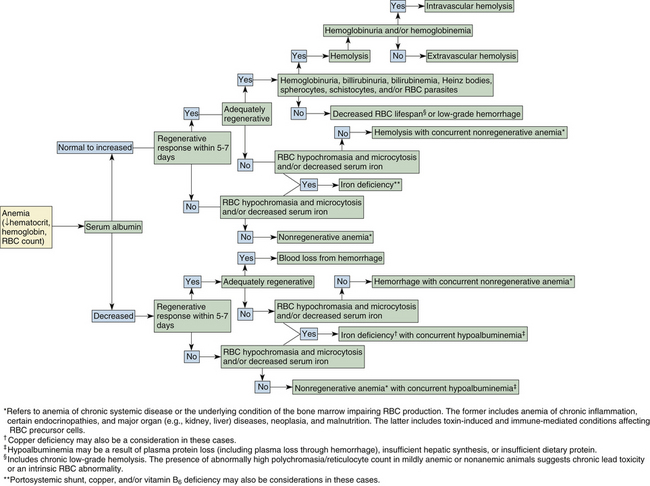
Anemia is an especially common finding in dogs and cats and may be secondary to almost any type of illness. Anemia is often suspected before blood samples are collected on the basis of a patient’s clinical signs and physical examination. Evidence to support the condition as being acute or chronic may be derived from the clinical presentation and history. An animal with per-acute to acute blood loss is often anxious and tachypneic and may have mucous membranes that are paler than expected for the degree of anemia as a result of transient peripheral vasoconstriction. With chronic blood loss, through upregulation of RBC 2,3-diphosphoglycerate in dogs and likely by an alternative mechanism in cats, oxygen is more readily released from hemoglobin to the tissues. Animals with chronic anemia are apt to be relatively inactive and show distress and dyspnea only if further stressed by physical exertion, an additional medical condition, or if the blood loss is severe. A dog or cat presenting with a packed cell volume (PCV) of 12% or less typically has some degree of chronic anemia because acute or subacute blood loss to this magnitude is generally not life supporting. Blood smear examination, as described in the following section, and in Figure 26-21, may further aid in determining the cause of anemia in dogs and cats.
Figure 26-22 An new methylene blue (NMB)–stained blood film from a cat.
Both aggregate and sparsely stippled punctate reticulocytes are seen in this field. (NMB stain. Original magnification 250×.)
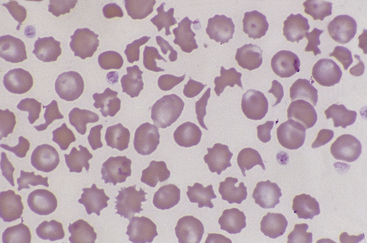
Figure 26-23 Many fragmented red blood cells are seen in this blood smear from a dog with stenosis of the pulmonic valve. Numerous schistocytes and a helmet-shaped cell are seen. (Wright stain. Original magnification 250×.)
Blood Smear Examination in the Evaluation of Anemia
Cytologic examination of peripheral blood is important in determining the cause, treatment, and prognosis of a patient’s anemia. The procedure is also valuable in monitoring anemic conditions over time. Alterations of RBC morphology are usually most indicative of the primary cause of the anemia. For example, anisocytosis may be detected in animals with regenerative erythropoietic response or immune-mediated hemolysis with spherocytic RBC and is especially profound when the two conditions are concurrent (Figure 26-25 and Figure 26-27). An increase in the proportion of large, immature RBCs over time without a change in absolute RBC count suggests that an animal has persistent blood loss (or hemolysis) and a responsive marrow. Other components of the smear, including leukocytes and platelets, may also provide clues about the cause of anemia. Increased numbers of normal or enlarged platelets may be seen in association with acute or persistent blood loss. Low platelet numbers support platelet consumption or destruction, with anemia secondary to hemorrhage or immune-mediated hemolysis. Leukocytosis is usually also seen with immune-mediated red cell destruction, whereas leukopenia (especially neutropenia) and thrombocytopenia occur concomitant with anemia with impaired bone marrow hematopoiesis.
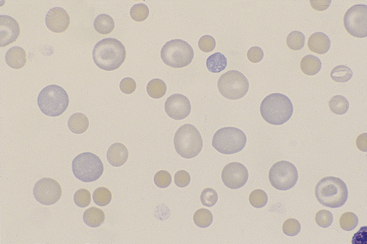
Figure 26-25 A blood smear from a dog with immune-mediated hemolytic anemia.
Note the marked anisocytosis resulting from a mixed population of small spherocytes and large, immature, and normal red blood cells. The spherocytes are dense staining and lack central pallor. A few polychromatophils are in the shape of codocytes. (Wright stain. Original magnification 250×.)
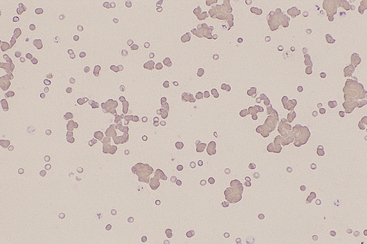
Figure 26-26 Red blood cell agglutination is seen in this blood film from a dog with immune-mediated hemolytic anemia. The cells remained agglutinated after saline dilution of the blood. (Wright stain. Original magnification 100×.)
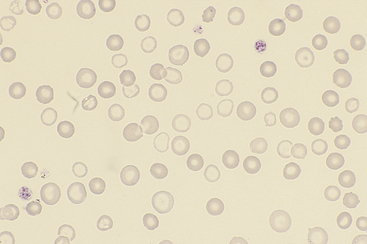
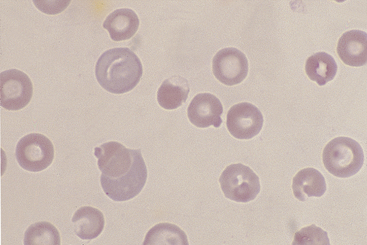
Figure 26-27 Blood from a dog with chronic blood loss and severe iron deficiency.
Iron-deficient red blood cells have a broad area of central pallor and a thin rim of stained cytoplasm. Anisocytosis is also apparent. (Diff-Quik stain. Original magnification 132×.)
Reticulocyte Evaluation and Quantitation
Method
Canine reticulocytes are of the aggregate type, recognized by their dark blue, interlacing network of cytoplasmic precipitate. Feline reticulocytes are of two readily recognizable types: (1) aggregate reticulocytes (as in dogs) and (2) punctate reticulocytes (Figure 26-22). Punctate reticulocytes lack the reticular pattern of cytoplasmic staining but contain a few scattered, variably sized, dark-blue cytoplasmic granules. These two reticulocyte types are counted separately in feline blood because their kinetics during a regenerative response differ. Because feline punctate reticulocytes remain elevated longer (up to 2 weeks after resolution of anemia) than aggregate reticulocytes in peripheral blood, only aggregate reticulocytes are counted to assess an active regenerative response. Note that epierythrocytic Hemobartonella felis organisms, basophilic stippling, and drying artifacts on RBCs may appear similar to and may need to be differentiated from punctate reticulocytes on NMB-stained feline blood smears.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree