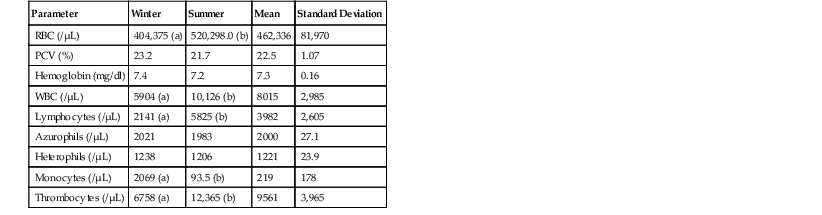
Brad A. Lock, Jim Wellehan Snakes are in the class Reptilia and the order Squamata, in the clade Toxicofera, infraorder Serpentes (snakes). More than 2900 extant species of snakes exist within three major clades: Scolecophidia, Alethinophidia, and Caenophidia.25,29 Scolecophidians are fossorial, blind snakes with three families in the group: Anomalepdidae (15 species [sp.]), Typhlopidae (210+ sp.), and Leptotyphlopidae (90+ sp.). Scolecophidians are oviparous and retain pelvic remnants. The Anomalepdidae (early blind snakes) live in the forested regions of Central and South Americas. The Leptotyphlopidae (Thread snakes) inhabit the semi-desert to forested regions of the tropics and subtropics of Africa, the Americas, and Southwest Asia. Thread snakes lack the left lung, the tracheal lung, and the left oviduct. The Typhlopidae (blind snakes) exist in areas ranging from the semi-desert to the rain forest regions throughout the tropics. The left lung is vestigial, and the left oviduct is absent. Ten families exist and comprise the Alethinophidian clade: Anomochilidae (2 sp.), Uropeltidae (45+ sp.), Cylindrophiidae (8 sp.), Aniliidae (1 sp.), Xenopeltidae (2 sp.), Loxocemidae (1 sp.), Boidae (40+ sp.), Pythonidae (25+ sp.), Bolyeriidae (2 sp.), and Tropidophiidae (23 sp.). Most of Alethinophidians have well developed, bilateral ovaries and retain both pelvic vestiges and hindlimb remnants as well as a left lung. Anomochilidae (false blind snakes) are fossorial and range from the Malay Peninsula, Sumatra, and Borneo. Uropeltidae (shield tail snakes) are fossorial species from Sri Lanka and India and possess no pelvic or hindlimb vestiges. Cylindrophiidae (pipe snakes) are fossorial snakes found in the forests of Sri Lanka, Southeast Asia, and the East Indies. The single member of the Aniliidae (false coral snake) is found in northern South America. Xenopeltidae (sunbeam snakes) are semi-fossorial species from the scrub and montane forests of Southeast Asia. Sunbeam snakes lack pelvic and hindlimb remnants. Loxocemidae (Mesoamerican python) is also semi-fossorial and inhabits the forested regions from Mexico to Costa Rica. The Boidae (boas) are a wide ranging group occupying fossorial, ground-dwelling, and arboreal habitats from the Americas, Central Africa, South Asia, Madagascar, the West Indies, and the Pacific Islands. Pythonidae (pythons) are restricted to the Old World and occupy diverse habitats in Africa, Asia, and Australia. Bolyeriidae (split jaw boas) possess a divided maxilla that has both anterior and posterior components, and this group is restricted to the island of Mauritius and its northern islets. Bolyeriids do not have pelvic or hindlimb remnants. Tropidophiidae (dwarf boas) do not have the left lung but are similar, anatomically, to boas and colubrids. This group is found in terrestrial and in semi-arboreal to arboreal niches in Malaysia, the Caribbean, and Central and South Americas. The Caenophidian clade is represented by five families: Acrochordidae (3 sp.), Atractaspididae (57+ sp.), Colubridae (1660+ sp.), Elapidae (195 sp.), and Viperidae (121+ sp.). Caenophidians are known as advanced snakes, and most of them have no hindlimb vestiges or left lung, but many species possess a well-developed tracheal lung. In the vipers, the oviducts are well developed bilaterally. Acrochodidae (file and wart snakes) are highly aquatic and range from South Asia to Australia. Viperidae (vipers and pit vipers) are all venomous and occupy all habitat niches worldwide except Papua-Australia, the oceanic islands, and Antarctica. Atractaspididae (stiletto or mole vipers) are venomous and occupy grassland and forested habitats from sub-Saharan Africa and the Arabian Peninsula. The Colubridae (king snakes, water snakes, bull snakes, etc.) make up the largest and most diverse group of snakes found throughout the world except Antarctica. Both venomous and nonvenomous species are found within this family, and they occupy a wide array of habitat types. The Elapidae (cobras, mambas, kraits) are venomous snakes that occupy a range of habitats throughout the Americas, Africa, Australia, Asia, and the Pacific Islands. In squamate evolution, the earliest divergence is the geckos, followed by the divergence of the skinks, night lizards, plated lizards, and girdled lizards. The next groups to branch off are the teiids, lacertids, and amphisbaenids, and the remaining group, comprising snakes, iguanids, agamids, chameleons, monitors, helodermatids, and anguids, is known collectively as the Toxicofera, named for the commonality of the presence of venom glands. Snakes diverge in the middle of the squamates, and if snakes are removed, lizards are not a monophyletic group. Snakes are a group of lizards, and a pine snake is a better model for a bearded dragon than a blue-tongued skink.25,39 With few and minor exceptions, the anatomy of the snake is consistent across species, and thus a general understanding of organ location can be developed.18 In general, the typical snake can be separated into three sections. The proximal one third of the snake contains the esophagus, trachea, parathyroid glands, thymus, thyroid, and the heart. The second third has the lung(s), continuation of the esophagus, liver, stomach, spleen, pancreas, gallbladder, proximal small intestine, and the air sac. The caudal third is the site for the caudal small bowel, gonads, adrenal glands, kidneys, cecum, colon, and cloaca. This system of thirds is often useful when attempting to identify an area of interest, when looking at a diagnostic image, or when deciding on a surgical approach. Snakes periodically shed their skins in one piece. This process is known as ecdysis, and the frequency is regulated by the thyroid gland. The integument of the snake is composed of two main types, scales and interscalar skin, both of which originate from the epidermis. Scales of both types (smaller scales on the dorsum and sides and larger ventral scales) are relatively inelastic and are composed of β-keratin, whereas the interscalar region composed of α-keratin is thin and highly elastic.21 The shed skin in snakes should generally come off in a single piece; however, larger and heavy-bodied species often shed in a few pieces. The entire process of ecdysis takes approximately 14 days to completion. Snakes do not have eyelids and instead have clear epidermis, the spectacle or brille, protecting the cornea. This spectacle is shed during ecdysis like the rest of the epidermis. Retained spectacles are a common finding with shedding or dysecdysis problems and have been associated with secondary infections of the cornea and adnexa of the eye. Normal snake skin is dry and warm and slightly cool to the touch when the animals are kept under proper husbandry conditions. Snake skin is dry because of the relative paucity of glands in the integument in most species. The well-known facial pit organs of vipers and many boas and pythons are extremely complex structures. These pits are true eyes and not just thermal receptors, which function not by photochemical reaction but on the basis of infrared and electromechanical radiation.10 Trigeminal innervations of the pit differ among groups of snakes possessing them, but both have innervations to the optic tectum of the brain and thus can form a usable image from this information.10 The skeletal system of snakes has a number of unique and interesting anatomic features. The skull is highly kinetic and does not have a rigid mandibular symphysis. The two rami of the mandible have a ligamentous connection. Snakes do not dislocate their jaws, as is commonly believed, but instead have an extra bone, the quadrate bone, connecting the lower jaw to the skull. This extra joint allows the snake to open the jaws approximately 180 degrees. These two anatomic features of the skull allow snakes to ingest extremely large prey items. Although snakes have retained ancestral cervical musculature, no cervical vertebrae are present (a snake has no neck).4,33 Almost all snakes possess pelvic remnants, but only the Alethinophidians have hindlimb vestiges, called spurs, which are often used to stimulate the female during copulation. The tongue is used in olfaction and is extended from an opening rostrally, called the filtrum. The tongue picks up chemical particles and then is returned to the mouth and contacts the Jacobson organ in the roof of the oral cavity. The Jacobson organ has direct connections to the olfactory nerves and serves to differentiate odors. Venom glands are modified salivary glands and combined with the highly evolved and derived venom delivery system used primarily for acquisition of prey and only secondarily for defense. Snakes only possess an inner ear structure, and the stapes bone is in direct contact with the quadrate bone and transmits vibrations allowing for the detection of low-frequency sounds (<600 hertz [Hz]).41 Thus, it has been a widely held belief that snakes only “hear” or respond to vibrations. Recent work has demonstrated that snakes do “hear” and respond behaviorally, to airborne sounds of various frequencies. They are able to perceive both airborne and groundborne (known as somatic hearing) vibrations.40,41 The glottis in snakes is located on the floor of the buccal cavity, and the trachea has incomplete rings. Snakes possess one or two lungs, with the more primitive groups—boas and pythons—having two, whereas the more advanced groups have only one lung (the right lung). When two lungs are present, the right one is the primary lung, and the left is reduced. Gas exchange occurs in the cranial compartment of the lung(s), and the caudal segment or air sac serves to store air and is used in defensive “puffing” displays. The cloaca is comprised of the coprodeum, the urodeum, and the proctodeum (CUP). The coprodeum is positioned dorsally and receives fecal material from the colon. The urodeum is located ventrally and receives urine and urate material from the ureters and ova and offspring from the oviducts. The proctodeum is the common collection chamber for the urodeum and coprodeum. The liver starts just caudal to the heart and ends at the cranial portion of the stomach. A gallbladder is present. However, the gallbladder is not associated with the liver but is positioned more caudally in the coelomic cavity, often near the pancreas and the spleen. The pancreas, spleen, and gallbladder can form a triad in some species, but in the majority of snakes, a combined spleno-pancreas is present. The gonads are located just cranial to the kidneys and are positioned asymmetrically, with the right organ being cranial to the left. The ovary can be extremely elongated, especially during the reproductive cycle. The kidneys are highly lobulated and usually dark in color. Male snakes have paired copulatory organs, called hemipenes, invaginated into the base of the tail. Female snakes can be oviparous (egg-laying) or viviparous (live bearing). Rattlesnakes in the past few years have been shown to display maternal care of offspring by remaining near the young until after the young have gone through a first shed. Female rattlesnakes have been seen to provide a refuge to the young. Rattlesnake neonates have been observed to seek out the mother when threatened, and mother rattlesnakes have been seen to actively move the young away from perceived danger. Temperature provision is an important husbandry parameter in the overall health and well-being of captive snakes. Snakes are ectothermic and depend on environmental temperature to regulate their core body temperature. A temperature mosaic (top to bottom, side to side, and front to back) should be established on the basis of the biology of the particular species being housed, using external infrared heat sources that create basking zones. In many species, a night time drop-in temperature is safe and necessary to maintain good health. Humidity within an enclosure is important to monitor in captivity. Environmental controls (HVAC) used to maintain building temperatures can cause enclosures to become excessively dry. Snakes maintained in environments with low humidity may be predisposed to chronic dehydration, dysecdysis, and chronic renal problems. In contrast, excessive humidity is associated with development of many forms of dermatitis. For enclosures not intended for public view, the author (BL) prefers the use of a humidity chamber to increase humidity. A simple humidity chamber can be created by placing moist sphagnum moss into a plastic container of the appropriate size, with an entrance hole for the snake. Ultraviolet (UV) lighting is generally not considered critical to the health of captive snakes. Recently, however, a study showed that certain snakes increase their circulating 25 hydroxyvitamin D3 concentration when exposed to UVB radiation.1 UVA and rich visible light, provided by full spectrum lighting, may be important to snakes for regulating behavior and for stimulating reproduction and feeding response. Venomous snakes should be housed in enclosures equipped with a locking mechanism, and the snake must be visible from outside. Large signs should be placed on the enclosure that indicate the presence of a venomous snake inside. An emergency snake bite protocol should be developed in conjunction with a local hospital. Many species of snakes have specialized diets of one or just a few prey items. Snakes are true carnivores, and no snake has been documented to be herbivorous. If possible, prey items should come from a commercial source. Although no guarantee exists, this will usually reduce the chances of parasite transmission compared with collecting road-killed or wild-captured prey. Freezing prey items for a few days prior to offering them as food to the snake will also help reduce parasite transmission. All mammalian and avian prey should be fed prekilled. The vast majority of captive snakes will either take prekilled prey immediately or quickly learn to accept prekilled food items. Live prey items, especially rodents, may cause extensive trauma to snakes if left alone in the enclosure. Frequency of feeding can be based on a number of factors. In general, juvenile snakes may be fed more often (every 5 to 10 days) compared with adult snakes (every 1 to 2 weeks). Clinically healthy snakes may also be fasted periodically for 4 to 8 weeks without harm. The reproductive status of both male and female snakes may alter the frequency of feeding as well. When cycling a female for the breeding season, more frequent feedings are often done to increase weight prior to the fasting period associated with the gravid state. Male snakes will often refuse food during the breeding season and may become anorexic for weeks or months. Snake species that consume fishes and amphibians will generally eat more often, and two to three times a week may be appropriate. Restraint of nonvenomous snakes can be accomplished by gently grasping the head of the snake immediately behind the mandible. The body of the snake is then supported by one or more handlers (one handler for each 3 to 4 feet of snake) to protect the spine. Venomous snakes should only be manipulated and restrained by trained professionals. These animals should be manipulated by using hooks and tongs and then restrained with the use of a clear plastic tube. The snake is encouraged to enter the tube to the midpoint level, at which time the snake’s body and the tube are grasped to prevent further advancement. This technique allows for safe management of the snake for physical examination, blood collection from the ventral coccygeal vein, and administration of parenteral or gas anesthetic agents. Anesthesia and analgesia (Tables 8-1 and 8-2) in snakes are used to facilitate surgery and other painful or invasive procedures and enhance the quality or safety of diagnostic sampling while minimizing stress and discomfort. Dissociative anesthetics, propofol, local anesthetics, and inhalant anesthetics are the most commonly used anesthetics in snakes. TABLE 8-1 Select Anesthetic Protocols for Snakes* * Maintenance for each is isoflurane or sevoflurane via endotracheal tube with or without nitrous oxide. TABLE 8-2 Selected Anesthetic and Analgesic Drugs and Dosages Used in Snakes The inhalant anesthetics (isoflurane, sevoflurane, and nitrous oxide) may be used to provide general anesthesia in snakes, isoflurane being the most common. The induction times and cardiorespiratory parameters of isoflurane and sevoflurane in snakes are very similar.6 Although no controlled studies have been performed in snakes, nitrous oxide used as a supplemental agent during induction in monitor lizards reduced the minimum alveolar concentration (MAC) by 25%.5 Apart from this reduction in the primary agent, which may improve cardiopulmonary function, the use of nitrous oxide likely provides improved analgesia, but this has not been critically evaluated in snakes. The primary benefit of the inhalant anesthetics over injectable agents is more direct control over the anesthetic during the procedure. At a surgical plane of anesthesia, most snakes will become apneic for long periods and should be intubated and provided with positive pressure ventilation six times per minute. Recovery should be on room air in a warmed, dark environment. Propofol is an alkylphenol in lipid suspension that may be used singularly to provide surgical induction of anesthesia. Propofol must be administered parenterally (intravenous [IV], intraosseous, intracardiac routes) and has rapid induction (1 to 5 minutes) and recovery (30 to 45 minutes) times in most cases.20 Ventilation is generally needed following propofol use. A recent study evaluating the histologic effects of intracardiac administration in snakes showed that lesions in cardiac tissues were mild and resolved after 14 days.20 Alfaxalone is a neuroactive steroid molecule with properties of general anesthesia.27 The effects of alfaxalone in a range of reptile species have been reported and include rapid induction with good muscle relaxation when given by the IV route but prolonged induction when given by the IM route. Recently, alfaxalone was evaluated in five snake species.27 Heavy sedation was achieved in 13 of 15 individuals tested at a dose of 9 milligrams per kilogram (mg/kg) IV alfaxalone (10 milligrams per milliliter [mg/mL]) and all 13 were easily intubated. The authors suggest that alfaxalone may be preferable to propofol as an injectable induction agent because of less cardiorespiratory depression, shorter induction times, and shorter total duration of anesthesia. The potent dissociative, tiletamine, combined with the benzodiazepine, zolazepam, continues to have some use in snake anesthesia. The onset of effects is faster than with ketamine, but variable effects are seen in snakes even at high doses (88 mg/kg).6 Despite the long duration of action and variable effects, the author sometimes uses low doses for sedation of large, aggressive snakes prior to handling or intubation. Compared with other classes of reptiles, very little is known about analgesia in snakes. Snakes seem to differ from the other lizards that have been investigated (primarily green iguanas and bearded dragons) and chelonians in that they show antinociceptive effects in response to the opioid butorphanol but not to morphine. In corn snakes, a butorphanol dose of 20 mg/kg significantly increased thermal withdrawal latencies 8 hours after subcutaneous (SQ) administration, whereas a lower dose of 2 mg/kg had no effect.29 The effective dose of 20 mg/kg is much higher than in other reptile species. Further investigation of snakes and other squamates is needed. Any presurgical workup for a snake should include the following: a complete physical examination, complete blood cell count (CBC), and plasma biochemistry. These will help assess the anesthetic risk and determine the suitability of the snake for surgery. Supplemental heat should be provided in all cases during the procedure. The coeliotomy is the most common surgical procedure performed by the author in snakes. Coeliotomy is performed to gain access for clinical evaluation and biopsy of internal organs and masses and to correct dystocia. Because a snake’s ventrum contacts the substrate and a large abdominal vein runs along the ventral midline, surgical approaches should not be made through the ventral scutes. Approaches to the coelomic cavity of a snake should be made between the second and third rows of lateral scales to prevent contamination of the incision by the substrate. Coeliotomy closure should be performed in two layers: the body wall and the skin. The skin is considered the holding layer and should be closed by using an everting suture pattern such as a horizontal mattress suture. An absorbable synthetic suture should be used to close the body wall, whereas nonabsorbable suture can be used to close the skin. In one study, polyglyconate and poliglecaprone were the least reactive of the materials.19 Sutures can be removed 4 to 8 weeks after surgery. Indications for gastrointestinal (GI) surgery in snakes include removal of foreign bodies, management of intestinal prolapse or intussusception, excision of masses or granulomas, and relief of impaction that generally involves the colon. Clinical signs associated with GI surgical diseases include weight loss, anorexia, abdominal distention, and vomiting or regurgitation. The basic principles of GI surgery in mammals apply to snakes. Special care should be taken when manipulating the thin-walled intestine of the snake. When closing incisions in the GI tract, it is generally considered best to get serosa-to-serosa contact.16 A two-layer closure is ideal to close the bowel, but this is often not possible in small snakes. If any concern regarding the integrity of the closure exists, a serosal patch should be applied. Any nearby organ that has a serosal surface such as an adjacent segment of bowel, the liver, and so on may be taken and tacked in place over the closed surgical site. The two serosal surfaces will rapidly adhere to each other, thus creating a seal. Once the bowel is closed, the coelomic cavity should be irrigated with warm, sterile saline. Broad-spectrum antimicrobials are indicated if contamination is suspected. Dystocia in snakes is generally postfollicular, and clinical signs include anorexia, regurgitation, straining, and cloacal discharge. Salpingotomy is indicated in patients that are reproductively valuable, where noninvasive techniques have failed, or if radiographic evidence indicates that natural passage is not possible. The surgical approach is a standard coeliotomy approach. When performing a salpingotomy in a snake, the incision should be made into a relatively avascular region of the oviduct. In snakes, it is often necessary to make more than one incision to access all eggs or fetuses, as these are commonly adhered to the friable oviduct. Once the procedure is complete, the incision in the oviduct should be closed by using a simple-continuous pattern with absorbable suture. Subspectacular abscesses are commonly seen and may occur as a result of ascending infections from the oral cavity. Because of the presence of the spectacle, topical treatment is unrewarding. Treatment requires excision of the spectacle to gain direct access to the subspectacular space. This procedure should be performed with the animal under general anesthesia. A small triangular wedge is excised from the ventral aspect of the spectacle to allow for drainage of purulent material. Samples for cytology and culture may be obtained from the site by using a sterile swab. The corneal surface is then irrigated with normal saline or ophthalmic solution to remove any material. A topical ophthalmic antibiotic is instilled three to four times per day to manage the infection. Systemic antimicrobials, based on culture and sensitivity, may be warranted in cases of panophthalmitis. Over time the spectacle will regenerate, but the original incision site must remain open until the infection is under control. In snakes, clinical signs of disease are often nonspecific (anorexia, lethargy), thus it is important to be able to use a variety of different diagnostic techniques and modalities to arrive at a diagnosis. The physical examination in the snake should always begin by observation from a distance without restraining the animal. Special attention should be given to tongue usage, respiration, and locomotion. The physical examination should be performed thoroughly and consistently. The spectacles should be clear, with no indication of retained epidermis or subspectacular disease. The nares should be clear and free of discharge and retained shed. The oral cavity should be evaluated by using a soft, pliable speculum. The mucous membranes should be pale to pink, without the presence of thick, ropey mucus. The glottis should be free of discharge, and the tongue should be inspected for function. The integument should be inspected closely for ectoparasites, traumatic injuries, and inflammation (dermatitis). The spine and ribs should be palpated; the spine is prominent in snakes with muscle wasting. The epaxial muscles should be well developed. Palpation should be performed to assess the coelomic cavity for abnormal masses present, and these should then be further evaluated by using appropriate diagnostic tests. Doppler ultrasonography may be used to determine heart rate and rhythm. Blood samples should be collected from the heart, the jugular veins, or the ventral coccygeal vein. Cardiocentesis is the most reliable venipuncture technique for snakes weighing more than 200 grams (g). Placing the snakes in dorsal recumbency facilitates visualization of the beating heart one fourth to one third the distance from the snout. The jugular veins are located cranial to the heart where the lateral and ventral scales meet. The snake should be placed in dorsal recumbency for the procedure. A 1- to 1.5-inch, 22-gauge needle attached to a 1- to 3-mL syringe should be inserted approximately nine ventral scales cranial to the heart on the medial aspect of the ribs. The ventral coccygeal vein is located ventral to the caudal vertebral bodies. A 1- to 1.5-inch, 22- to 25-gauge needle on a 1- to 3-mL syringe may be used to collect the sample. Using gravity can be very useful to collect the sample; tipping the snake up with the tail lower the head often causes pooling of blood in the tail. In male snakes, caution should be used to avoid the hemipenes in the tail base. GI lavage and pulmonary lavage may be performed to collect samples from snakes that are regurgitating or showing signs of pneumonia. A red rubber tube may be used to collect the sample. The tube should be premeasured to ensure correct placement into the stomach or lungs or through the cloaca into the colon. Physiologic saline (5 to 10 mL/kg, 3% to 5% body weight in milliliters) may be used to perform the lavage. Centrifugation of samples is often used to concentrate organisms before submission. Computed tomography (CT) has demonstrated usefulness in the diagnosis and monitoring of treatment for pneumonia in snakes, and a few papers have described normal CT anatomy.3,24 Ultrasonography may be used for survey imaging and as a guide for collection of diagnostic samples. Ultrasound-guided fine-needle aspiration of identified masses and fluid areas is often rewarding. Ultrasonography is well suited for monitoring the reproductive cycle of snakes, and this can be valuable in endangered species. Endoscopy, which is highly effective in snakes because of the elongated shape and dispensability, may be used to visually evaluate organs and collect diagnostic samples for culture, cytology, and histopathology. Blood cell counts and plasma biochemical values in snakes are affected by environmental conditions, reproductive status, season, location, and nutrition.2,7,8,15,26,34 Reference material for hematologic and plasma biochemistry in snakes is sparse and variable, making interpretation of a given sample difficult. Given this, it may be most efficient and effective to establish in-house reference ranges for groups or individual snakes. If this is done over a few years in different seasons and conditions, the significance of subsequent changes detected will be more easily interpretable. Blood volumes in reptiles vary from 5% to 8% of their total body weight. Of this amount, up to 10% may be safely collected for analysis without harm to the patient. Blood samples may be stored in EDTA (ethylenediaminetetraacetic acid) or lithium heparin microtainers. CBC may be performed on samples stored in either anticoagulant. Recently, it has been shown that mixing of the blood sample with 22% albumin (1 drop albumin to 5 drops of blood) will reduce white blood cell (WBC) damage during the smear process.30 Reference ranges for select snake species under different conditions are presented in Tables 8-3 through 8-10. TABLE 8-4 Hematology and Plasma Biochemistry Reference Values for Apparently Healthy, Free-Ranging, Giant Garter Snake (Thamnophis gigas) and Valley Garter Snake (Thamnophis sirtalis fitchi).
Ophidia (Snakes)
Biology
Taxonomy and Geographic Distribution
Unique Anatomy and Physiology
Special Housing Requirements
Feeding
Restraint and Handling
Anesthesia and Analgesia
Snake
Premedication
Induction
Small
None
Propofol (5–10 mg/kg, IV)
Direct intubation followed by ventilation with isoflurane or sevoflurane
Tube induction with isoflurane or sevoflurane
Large
Tiletamine/zolazepam
(5–10 mg/kg, IM), or
None
Propofol (2–7 mg/kg, IV)
Direct intubation followed by ventilation with isoflurane or sevoflurane
Venomous
None
Restraint in a tube followed by propofol (5–10 mg/kg, IV)
Tube or chamber induction with isoflurane or sevoflurane
Generic Name
Dosage (mg/kg)
Reversal Agent
Propofol
3–10
Midazolam
0.2–2
Flumazenil
Butorphanol
20 (Monitor for respiratory depression)
Tiletamine/zolazepam
2–10
Flumazenil for zolazepam
Mac %
Isoflurane
1.5–2.1
Sevoflurane
2.5
Nitrous oxide
220
Surgery
Diagnostic Sampling and Physical Examination
Hematology
Parameter
N (T. gigas)
N (fitchi)
Median (T. gigas)
Median (T. fitchi)
Range (T. gigas)
Range (T. fitchi)
WBC (×10–3/µL)
46
35
11.5 (a)
6.6 (b)
2.5–18.6
3.1–13.7
RBC (×10–6/µL)
46
36
0.8
0.9
0.2–1.4
0.5–1.3
PCV (%)
46
37
31
29.5
17–45
18.5–42
Heterophils (×10–3/µL)
45
37
0.99 (a)
0.59 (b)
0.35–2.18
0.23–3.70
Lymphocytes (×10–3/µL)
46
37
7.9 (a)
4.27 (b)
1.27–14.97
1.66–15.10
Basophils (×10–3/µL)
45
37
0.33
0.29
0.09–0.83
0.65–0.86
Azurophils (×10–3/µL)
44
36
1.75
0.73
0.37–4.4
0.19–1.94
Plasma protein (g/dL)
45
35
5
5.5
4.2–6.7
4.0–8.3
AST (IU/L)
44
34
22
16
8–74
8–48
Bile acids (µmol/L)
45
34
35
35
0–35
35–47
Creatinine kinase (IU/L)
42
35
439
387
20–1666
17–1428
Uric acid (mg/dL)
44
33
5.7
5.1
1.2–13.1
1.7–16.0
Glucose (mg/dL)
43
33
81
89
44–154
53–167
Calcium (mg/dL)
45
33
15.2
15.7
12.9–20.0
11.7–16.6
Phosphorus (mg/dL)
43
34
3.8
3.6
1.9–6.3
1.8–7.6
Total protein (g/dL)
44
35
5
5.2
3.9–6.1
4.1–7.9
Albumin (g/dL)
45
36
1.2
1.2
1.0–1.7
1.0–2.1
Globulin (g/dL)
43
36
3.6
4.0
2.7–4.7
0–7.0
Potassium (mmol/dL)
44
36
5.2
4.4
2.7–8.8
1.8–7.0
Sodium (mmol/dL)
44
35
159
157
147–170
136–166 ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Ophidia (Snakes)
Chapter 8