Significance of Metabolic Stress, Lipid Mobilization, and Inflammation on Transition Cow Disorders
Lorraine M. Sordillo, MS, PhDa∗ and William Raphael, BVSc, MSb, aDepartment of Large Animal Clinical Sciences, College of Veterinary Medicine, Michigan State University, 784 Wilson Road, G300 Veterinary Medical Center, East Lansing, MI 48824, USA; bDepartment of Large Animal Clinical Sciences, College of Veterinary Medicine, Michigan State University, 736 Wilson Road, D202 Veterinary Medical Center, East Lansing, MI 48824, USA. E-mail address: sordillo@msu.edu ∗Corresponding author.
Keywords
Inflammation
Lipid mobilization
Oxidative stress
Transition period
Disease
Key points
Dairy cattle are susceptible to increased incidence of metabolic and infectious diseases during the physiologic transition from late pregnancy to early lactation. Dramatic changes in both metabolic activity and dysfunctional immune responses are closely associated with the development of many transition cow disorders.1,2 Although dairy cows undergo several physiologic changes during the onset of lactation that may contribute to health problems, the mobilization of excessive body fat reserves and significant increases in plasma fatty acid concentration are important risk factors leading to enhanced disease.3 The direct role that increased lipid mobilization has on liver function and the pathogenesis of certain metabolic diseases such as fatty liver and ketosis is well established. However, more recent evidence suggests that increased plasma fatty acid concentrations may indirectly affect both metabolic and infectious disease pathogenesis by compromising the function of cells involved in immune responses. Uncontrolled inflammatory reactions are especially important in the pathogenesis of several transition cow disorders. A better understanding of the interrelationship between metabolic stress, lipid mobilization, and immune dysfunction during the transition period facilitates the design of better control programs to prevent health disorders during this critical period in the production cycle of dairy cows. This article addresses the possible linkages between fat mobilization and dysfunctional inflammatory responses that may contribute to increased morbidity and mortality in transition dairy cows.
Physiologic adaptations of the transition cow
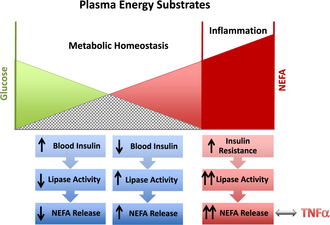
The transition period for dairy cows is defined as approximately 3 weeks pre partum until 3 weeks post partum. Major physiologic, nutritional, metabolic, and immunologic changes occur within this time frame as the production cycle of the cow shifts from a gestational nonlactating state to the onset of copious milk synthesis and secretion. For example, cows must adjust metabolically to the dramatic increase in energy requirements that is needed to ensure optimal milk production in the ensuing lactation. Milk production requires large amounts of carbohydrates for lactose synthesis, and nearly all the available glucose in the body is diverted to the mammary gland for this purpose. Dairy cattle experience appetite suppression during the last week of gestation, and it can take up to a week after calving before dry matter intake (DMI) recovers.4 The imbalance between energy consumed and the energy needed for production demands is termed negative energy balance (NEB). During times of NEB, sufficient energy must be mobilized from tissue stores to support energy-dependent needs of the body, and adipose tissues are a major fuel source for cows during the transition period. However, a balanced metabolic response during the onset of lactation is needed to regulate the appropriate amount of lipid mobilization. Initially, a decrease in blood glucose level occurs in response to both high demands of lactation and diminished DMI. The reduction in blood glucose results in lower insulin levels, which trigger the fat mobilization process through lipolysis. During lipolysis, nonesterified fatty acids (NEFAs) are cleaved from triglyceride molecules within adipocytes through the action of various hormone-sensitive lipases. NEFA is then transported into the blood by albumin, where it can be used as an energy source and also initiate negative feedback loops to regulate the amount of lipolysis (Fig. 1). The overall cumulative effect should result in relatively constant blood glucose concentrations needed for milk synthesis and secretion without excessive NEFA accumulation in the blood.5
Metabolic Stress
Cows successfully adapt to NEB when adipose mobilization is adequately regulated and the release of NEFA is limited to concentrations that can be fully metabolized for energy needs. Although lipid mobilization provides the energy needed to promote milk production, excessive release of lipids from adipose tissues and accumulation of high concentrations of free fatty acids in the blood are positively correlated to several metabolic problems in the transition cow.6 The development of ketosis and fatty liver, for example, is a direct consequence of significant increases in plasma NEFA concentrations.5 Recent studies showed that multiparous cows with plasma NEFA concentration higher than 0.57 mEq/L after calving experienced a 600-kg decrease in milk yield.6 Reduced lactation performance as a consequence of high plasma NEFA concentrations can be explained, in part, by the detrimental impact on liver function. When taken up by hepatocytes, NEFA can be re-esterified to triglycerides or undergo β-oxidation to generate energy in the mitochondria. However, during intense NEFA flux, the capacity of hepatocytes to export newly synthesized triglycerides is exceeded. Excessive triglyceride accumulation in the liver also results in reduced fatty acid oxidation, which is essential for gluconeogenesis. A reduction in gluconeogenesis in the liver as a result of triglyceride accumulation limits the available blood glucose needed for optimal milk production and contributes further to the energy deficits in the transition cow. The uptake of NEFA in the liver also results in the production of ketone bodies as intermediate products during fatty acid metabolism. Ketones are released into the blood, where they can be used as energy substrates, especially in muscle and nerve tissues. Ketosis develops during intense lipid mobilization when the rate of ketogenesis exceeds ketone body use. The most abundant ketone body, β-hydroxybutyrate (BHB), is often used to assess the degree of NEB and lipid mobilization in transition dairy cows.
Overconditioned cows with high body condition scores (BCSs) were reported to have a greater incidence of health problems in the transition period when compared with leaner cows.7 Cows with a high BCS tend to lose more body weight through lipid mobilization, resulting in enhanced increases in NEFA and BHB concentrations during the onset of lactation. In humans, obesity is associated with insulin resistance, and the large adipose mass becomes less responsive to the modulating effects of insulin.8 Thus, obesity may exacerbate the severity of metabolic diseases by the formation of destructive feedback loops that lead to enhanced lipolysis and increased blood NEFA and BHB concentrations (see Fig. 1). Although the potential of obesity-induced insulin resistance is not established in cows, there is a model indicating that this may be the case,9 and excessive body fat is a well-established positive risk factor for metabolic disorders in cows.3
Inflammatory Response
Intense adipose mobilization may also be a critical factor leading to dysfunction immune responses and infectious diseases during the transition period. Significant increases in lipid mobilization predispose dairy cows to both metabolic (fatty liver, ketosis) and infectious (mastitis, metritis) diseases.10,11 The transition period is characterized as a time of dramatic changes in the efficiency of the bovine immune system, which coincides with metabolic stress. Dairy cattle defend themselves from invading pathogens by a complex network of immune cells and soluble mediators, which are highly coordinated to provide optimal disease resistance. Inflammation is a critical component of the initial immune response, which often determines if pathogens are able to establish disease. The inflammatory process is initiated when localized immune and nonimmune cells within the affected tissues are able to recognize invading microbes through highly conserved pathogen recognition receptors. The recognition process activates these resident cell populations and stimulates the release of potent proinflammatory signaling molecules, including nitric oxide, eicosanoids, and cytokines. These inflammatory mediators can act locally on the vascular endothelium to increase blood flow and facilitate the migration of leukocytes from the blood to the site of infection. Newly recruited and preexisting leukocytes, primarily neutrophils and macrophages, act cooperatively to eliminate microbial pathogens through phagocytosis and various intracellular killing mechanisms. Cytokines and eicosanoids can also elicit systemic inflammatory responses, which include release of acute phase proteins from the liver, increased body temperature, increased heart rate, and reduced feed intake. An efficient inflammatory response eliminates the invading pathogen, restores immune homeostasis, and returns tissues back to normal function and morphology.
Many aspects of the bovine immune system are compromised around the time of calving, especially with respect to appropriate inflammatory responses.12 There are several ways that excessive or chronic inflammatory responses can lead to increased health disorders in the transition cow. During infectious diseases, for example, macrophages and neutrophils are important for the prompt detection and elimination of invading pathogens. Many studies have documented that the antimicrobial functions of these leukocyte populations are compromised and certain pathogens are able to evade local defenses and establish long-term disease. Mastitis caused by either Staphylococcus aureus or Streptococcus uberis are examples of how widespread immune evasion of mammary gland immune cells can result in chronic inflammation and extensive tissue damage. Conversely, there are some mastitis-causing pathogens that can elicit uncontrolled recruitment and activation of inflammatory cells, which contribute to pathogenesis. Coliform mastitis is more severe during early lactation when compared with later stages of lactation. Early lactation cows with coliform mastitis express high milk and serum concentrations of tumor necrosis factor α (TNF-α), interleukin 1 (IL-1), and IL-6, which are directly related to increased disease severity. The major sources of these proinflammatory cytokines are macrophages and endothelial cells. Previous studies showed that these cells are more responsive to bacterial endotoxins during early lactation, which may contribute to exacerbated inflammation responses during coliform mastitis.13,14 As a consequence, the delicate balance between a sufficient inflammatory response needed for optimal pathogen clearance and the prompt return to immune homeostasis is often lost during the transition period. Therefore, factors that contribute to either hyporesponsive or hyperresponsive inflammatory responses likely contribute to the pathophysiology of any inflammatory-based disease.
Link between metabolic and inflammatory responses
In human medicine, there is ample evidence to suggest that obesity and the associated increase in plasma NEFA concentrations lead to a chronic inflammatory condition.15,16 Systemic inflammation during obesity is characterized by the abnormal production of proinflammatory cytokines (ie, TNF-α and IL-6) and bioactive lipid mediators, which orchestrate the magnitude and duration of the inflammatory response. Adipocytes and macrophages that reside in adipose tissues are the cellular sources for these proinflammatory mediators and the resulting chronic inflammatory condition is believed to sensitize the body to both infectious and metabolic diseases in obese people.15 Increased TNF-α concentrations also can block downstream signaling of insulin-mediated events. In humans, TNF-α is believed to be the link between obesity-induced insulin resistance and increased lipid mobilization.16 The possibility that an overly aggressive or chronic inflammatory response during NEB can increase the incidence of transition cow disorders has been the subject of intense research interest.
Numerous studies suggest that certain aspects of energy metabolism, especially lipid mobilization, can negatively affect a balanced inflammatory response in transition dairy cattle. Dysfunction or unregulated inflammatory responses are believed to be the common link between the increased incidence of both metabolic and infectious diseases during the transition period. This assumption is based on the observations that metabolic and infectious diseases tend to occur in complexes with each other rather than as isolated events in cows during early lactation.17 Moreover, increased incidence of any single transition cow disorder increases the chance that they succumb to other health issues. For example, epidemiologic studies indicated an association between the development of retained placenta and the incidence of mastitis.18 In addition, cows suffering from ketosis were twice as likely to develop mastitis than healthy cows.19 Although a direct causal link has not been established in cattle, there is ample evidence to suggest that increased health disorders during the transition period are symptomatic of a dysfunctional immune system.
Impact of Metabolic Stress on Inflammation
The relationships between metabolic factors and compromised immunity during the transition period have been investigated extensively.2,20 Intense lipid mobilization during the transition period has long been recognized as a major contributing factor leading to immune dysfunction. The direct impact that metabolic stress has on immune functions around the time of calving was clearly shown using a mastectomy cow model.21 In this study, pregnant dairy cows were mastectomized to assess the impact of milk production and NEB on immunity, but presumably maintaining the endocrine changes associated with late pregnancy and parturition. The mastectomy cows did not experience the dramatic shift in NEB, as indicated by only moderate increases in NEFA when compared with the intact cows. The functions of lymphocytes and neutrophils were significantly better in the mastectomy cows when compared with the intact cows during the transition period. The major conclusion from this study was that increased lipid mobilization caused by the metabolic demands of lactation can directly diminish the antimicrobial functional capabilities of immune cells during early lactation.21 Another study22 showed that certain metabolites associated with NEB also can negatively affect neutrophils and other immune cell functions. Ketotic cows are believed to be more susceptible to mastitis and other infectious conditions because of the adverse effects that BHB concentrations have on leukocyte antimicrobial mechanisms.22,23 Plasma BHB concentrations are also significantly higher and neutrophil antimicrobial function lower during clinical cases of metritis.20 Another important metabolic adaptation that may affect immunity is the change in glucose availability during the transition period as a consequence of NEB. Macrophages and neutrophils require considerable energy to support their antimicrobial functions, and glucose serves as a primary fuel source.24 Therefore, the dramatic decrease in blood glucose concentrations during intense lipid mobilization and ketosis may also affect host defenses around the time of calving by limiting the fuels needed by immune cell populations.22 The concept that an activated inflammatory reaction may compete with other production-related processes (ie, milk synthesis) for limited nutrients may account for the decreased productive efficiency of dairy cows during morbidity.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree