Chapter 5. Non-surgical trauma
CHAPTER CONTENTS
The relative importance of ocular trauma in a mail-in pathology practice81
Proptosis and optic nerve trauma95
THE RELATIVE IMPORTANCE OF OCULAR TRAUMA IN A MAIL-IN PATHOLOGY PRACTICE
The relative importance of non-surgical trauma is hard to estimate:
Comparative Comments
• It is the third most common reason for enucleation in dogs and cats, behind glaucoma and neoplasia
• It is estimated that 20% of canine submissions to COPLOW are related to non-surgical trauma.
Comparative Comments
Non-surgical trauma is the leading cause of enucleation in the experience of the University of Wisconsin Eye Pathology Laboratory. It is estimated that about 20% of the human eye submissions are related to non-surgical trauma.
Yanoff and Fine (2002) state that trauma (both surgical and non-surgical combined) accounts for 35% of all enucleations.
GENERAL POST-TRAUMATIC RESPONSE OF OCULAR TISSUES, REGARDLESS OF THE TYPE OF TRAUMA
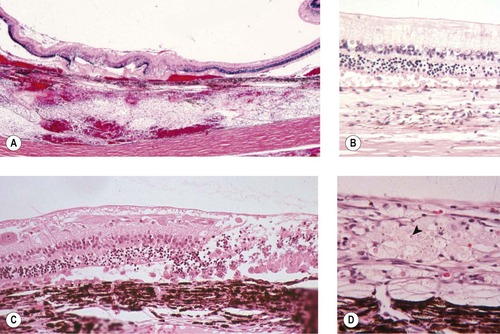
Non-specific proliferative reaction (Figure 5.1, Figure 5.2 and Figure 5.3)
• Within a few hours of trauma, cellular proliferation may be recognized in the lens epithelium and the non-pigmented ciliary body epithelium (Fig. 5.1)
 |
| Figure 5.1 Cellular effects of trauma. (A) Gross photograph of a feline globe removed 3 h after blunt trauma showing lens capsule rupture and retinal tear. (B) Photomicrograph showing the lens capsule and lens epithelial cell proliferation and spindle cell metaplasia in a feline globe removed 8 h after a traumatic event. (C) Photomicrograph showing the non-pigmented ciliary epithelial cells proliferating in a canine globe removed 5 h after a traumatic event. |
• After about 48 h of trauma, spindle cell and neovascular proliferation extend into the aqueous or vitreous compartments of the globe (Fig. 5.2) (see also Ch. 9)
▪ Neovascular proliferation
– May contribute to subsequent intraocular hemorrhage, potentially setting up a vicious cycle
▪ Pre-iridal fibrovascular membrane
– May lead to peripheral anterior synechiae that, in turn, contributes to the development of neovascular glaucoma
▪ Cyclitic membrane
– This can lead to traction resulting in retinal detachment
○ Retinal detachment and subsequent hypoxia leads to the release of growth factors which further stimulate neovascular proliferation
▪ Choroidal neovascular membranes (Fig. 5.3)
– This can lead to subretinal hemorrhage or serous exudation, with subsequent retinal detachment
 |
| Figure 5.3 Fibrovascular proliferation in the globe. (A) Photomicrograph showing a choroidal neovascular proliferation in a traumatized canine globe. (B) Photomicrograph showing a fibrovascular membrane extending from the optic nerve head into the vitreous body in a traumatized dog globe (arrow). (C) Low magnification photomicrograph of a canine globe showing extensive intravitreal hemorrhage and neovascular proliferation near the optic nerve head. |
 |
| Figure 5.2 More cellular effects of trauma. (A) Gross photograph of a canine globe showing broad anterior synechiae and developing cyclitic membrane (arrow). (B) Gross photograph of a canine globe showing posterior synechia (arrows) and iris bombé. (C) Photomicrograph of the anterior surface of the iris showing a pre-iridal fibrovascular membrane in a canine globe after trauma. |
▪ Neovascular membranes extending from the optic disk into the vitreous in dogs
– This can lead to traction and retinal detachment, with retinal hypoxia contributing to further neovascular proliferation
▪ Retinal neovascular membranes rarely occur in domestic animal species
Comparative Comments
Comparative Comments
Proliferative vitreoretinopathy occurs in humans secondary to trauma, or can be secondary to retinal detachment, and is rare in domestic species
▪ The general absence of granulation tissue formation within the tissues of the uveal tract is quite striking
– If fibrosis is observed within the uvea, it is important to look for evidence of scleral rupture, which provides a portal for fibrosis originating within the episcleral tissues to enter the globe.
Comparative Comments
The post-traumatic reaction of ocular tissues described for animal eyes applies also for human eyes.
INTRAOCULAR HEMORRHAGE
Blood in the anterior chamber (hemophthalmos) (Fig. 5.4)
• May result from blunt or penetrating trauma, but has many other potential causes; including intraocular inflammation, neoplasia, fibrovascular membranes, retinal detachment, systemic hypertension or disorders of hemostasis
• Hemophthalmos may play a role in stimulating a neo-vascular proliferative response which can lead to anterior or posterior synechiae, as well as predisposing to further episodes of intraocular hemorrhage
• In its aftermath, hemophthalmos can lead to secondary hemosiderosis
▪ Blood pigment staining of the cornea, after traumatic hemorrhage, can involve hemoglobin, hemosiderin, or hematoidin. Staining is seen most often in the peripheral cornea and the tissues of the iridocorneal angle. A Prussian blue stain is useful to distinguish hemosiderin from melanin
• Continued hemorrhage can fill the globe (hemophthalmos)
▪ Recurrent episodes of intraocular hemorrhage may reflect underlying disease; vicious cycles of hemorrhage associated with clotting and subsequent activation of the fibrinolytic pathway, or leakage from new vessels formed as part of a fibrovascular response
• Cholesterolosis bulbi (synchysis scintillans, cholesterol granuloma)
▪ Blood which pools within the globe and is not resorbed will eventually be broken down to hemosiderin and cholesterol, stimulating a profound phagocytic response. Frequently, free cholesterol crystals are identifiable within the globe as acicular cholesterol clefts in tissues or free in the chambers of the globe. Cholesterol crystals are birefringent when viewed with polarized light, but only if the tissues are unprocessed.
Comparative Comments
The occurrence and sequelae of intraocular hemorrhages are little different in humans from those described in animal eyes.
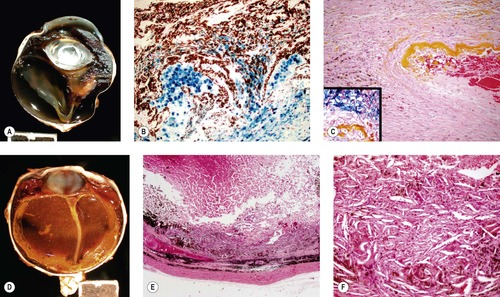 |
| Figure 5.4 Effects of hemorrhage. (A) Gross photograph of a canine globe with retinal detachment and accumulation of blood pigments in a nodular lesion that distorts the peripheral retina and vitreous body. (B,C) Photomicrographs of the lesion in (A) show hemosiderin stained with Prussian blue in (B) and in the inset in (C). Yellow hematoidin pigment appears in (C). (D) Gross photograph of a canine globe with longstanding intraocular hemorrhage, hemosiderophage and cholesterol granuloma formation. (E,F) Low magnification and higher magnification photomicrographs, respectively of the same globe as (D), show a loose accumulation of macrophage cells and abundant cholesterol crystals indicated by the spindle-shaped empty spaces in (F). |
NON-PENETRATING (BLUNT) OCULAR TRAUMA
Corneal effects
• Abrasion – not often a feature of a pathology submission
• Ruptures in Descemet’s membrane
▪ May lead to striate keratopathy
▪ Relatively common clinical presentation in horses and may follow blunt trauma, such as whip or rope injuries to eye, although a history of trauma is often lacking
▪ Careful evaluation is required to exclude glaucoma, which may cause of ruptures in Descemet’s membrane related to globe stretching (see Ch. 13)
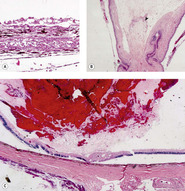
Uveal effects (Figs 5.5, 5.6)
• Cyclodialysis, the tearing and separation of the ciliary body and iris base from the sclera creating an opening into the suprachoroidal space (Fig. 5.5)
▪ Cyclodialysis is relatively more likely to occur in cats than dogs
– This is because of the absence of a robust primary pectinate ligament structure in the feline iridocorneal angle
▪ Cyclodialysis is relatively even more likely to occur in birds for the following reasons:
– The rigid scleral ossicles of the avian eye magnify shearing forces, which act to strip the soft tissues of the ciliary body away from the sclera
– The attachment apparatus at the iridocorneal angle is delicate
▪ Cyclodialysis is recognized in the acutely traumatized eye by paying close attention to the loss of integrity of the ciliary cleft and the tissues around the ciliary cleft
– However, years after the traumatic event, ‘angle recession’ may still be observed as evidence of prior trauma to the structures of the irido-corneal angle (Fig. 5.6)
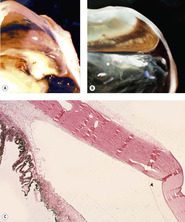 |
| Figure 5.6 Iridocorneal angle recession. (A,B) Gross photographs of two feline globes show a wide space between the end of Descemet’s membrane and the displaced iridocorneal angle (angle recession). (C) Low magnification photomicrograph of a feline globe showing an expanded distance between the end of Descemet’s membrane (arrow) and the iridocorneal angle in angle recession. |
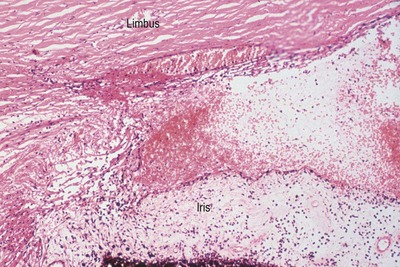 |
| Figure 5.5 Acute cyclodialysis. Photomicrograph of a feline iridocorneal angle within hours of blunt trauma showing hemorrhage in the anterior chamber and iridocorneal angle, as well as tearing away of the iris and ciliary body from the sclera (cyclodialysis). |
• Angle recession is characterized by the dramatically increased distance between the end of Descemet’s membrane and the anatomic irido-corneal angle. Angle recession has implications in aqueous drainage and is a risk factor in the development of glaucoma (see Ch. 13)
▪ In some cases, Descemet’s membrane extends over the inner sclera beyond the limbus and, in those cases, angle recession is recognized by the increased distance between the anatomic angle and the limbus.
• Rupture or avulsion of corpora nigra is often seen after blunt trauma in horses.
Lens effects (see also Ch. 10)
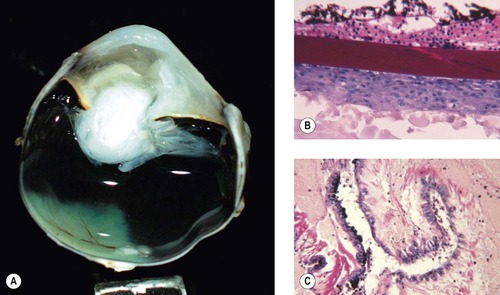
Cataract (Fig. 5.7)
Cataract is a common consequence of blunt trauma. Cataract can be prominent within days of trauma (Fig. 5.7).
• Anterior or posterior subcapsular cataract
• Cortical cataract, focal or complete, is often seen and it is good to search for other morphologic indicators of trauma such as retinal detachment or retinal necrosis/atrophy.
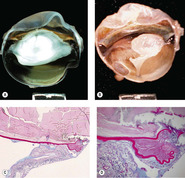 |
| Figure 5.7 Traumatic cataract. (A,B) Gross photographs show trauma-induced cataracts in a cat (A) and a dog (B). There is lens capsule rupture, displacement of lens fiber protein, and synechiae in both globes. (C,D) Low magnification and higher magnification of a canine post-traumatic cataract show lens capsular rupture and remodeling. |
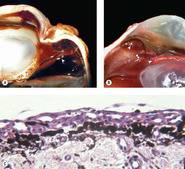
Lens capsule rupture (Figure 5.8, Figure 5.9 and Figure 5.10)
Lens capsule rupture is a commonly observed consequence of non-penetrating injury to the globe, in submissions to the pathology laboratory.
Pathologic lens capsule rupture
Pathologic lens capsule rupture is differentiated from artifact by the following findings (in descending order of diagnostic value) (Fig. 5.8):
• Presence of inflammatory cells such as macrophages or neutrophils, or blood vessels, inside the lens capsule
▪ This feature may be encountered, even if the actual lens capsule defect has not been sampled in the section
• Presence of a cellular reaction at the severed margins of the lens capsule
▪ This reaction can involve proliferating lens epithelial cells, inflammatory cells, or spindle cells and may be associated with synechiae
• Recoil or scrolling of the severed ends of the lens capsule.
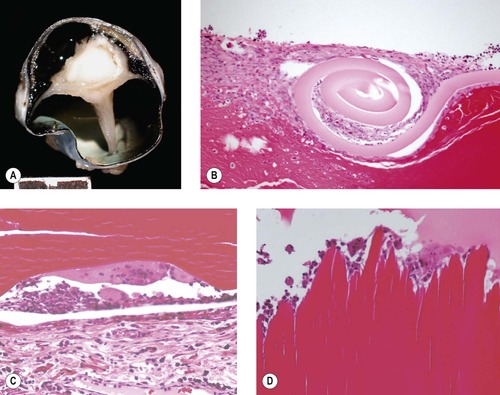 |
| Figure 5.8 Lens capsule rupture. (A) Gross photograph of a canine globe showing a small lens remnant and adhesion of iris, ciliary body, and detached retinal tissue. (B) Photomicrograph of a feline globe after traumatic lens capsule rupture showing coiling of the capsule and cellular proliferation on both sides of the capsular defect. (C,D) Photomicrographs showing macrophage and multinucleate giant cell reaction to exposed lens proteins, which is a reliable indication of lens capsular rupture. |
Inflammation
Inflammation (phacoclastic uveitis) is an important consequence of lens capsule rupture (Fig. 5.9).
• Immediately adjacent to the released lens material, there will be a foreign body granulomatous reaction
▪ Some authors assert that lens proteins released following capsule rupture frequently elicit a robust pyogenic or pyogranulomatous response. However, one must be cautious in distinguishing the effects of sepsis; which would be expected to elicit a pyogenic reaction, and may be introduced by a penetrating injury; from the response induced solely by exposure of the lens proteins, which is typically a relatively ‘bland’ foreign body, granulomatous reaction
• Away from the immediate area of exposed lens protein, a lymphocytic and plasmacytic uveitis develops, that may be associated with synechiae or neovascular membrane formation
• Phacoclastic uveitis often results in secondary glaucoma, due to the formation of synechiae or neovascular membranes.
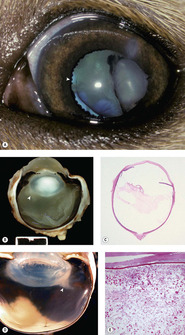 |
| Figure 5.9 Lens-induced uveitis, phacoclastic uveitis. (A) Mixed Breed, 12 weeks old: prior trauma resulted in lens rupture, cataract formation, and uveitis. Pigment is present on the lens and ectropion uvea is indicated by the arrow. (B) Vitreous protein exudation is adjacent to the ruptured lens capsule (arrow) in a traumatized dog globe. (C) Subgross photomicrograph of a canine globe showing a nearly empty lens capsular bag and protein exudates in the vitreous body secondary to the release of denatured lens proteins. (D) Gross photograph of a feline globe showing distinctive protein exudates (arrow) in the vitreous body near the posterior pole of the lens, which is associated with posterior lens capsule rupture and lymphoplasmacytic uveitis. (E) Photomicrograph of the globe in (D) showing the granular hypereosinophilic protein characteristic of posterior lens capsular rupture in feline uveitis. |
Spindle cell metaplasia of lens epithelial cells and migration of lens epithelial cells (Fig. 5.10)
• Spindle cell metaplasia of lens epithelial cells is the first step in the formation of subcapsular cataract in response to trauma, and is associated with posterior capsular opacification after cataract surgery. It is an important feature of the intraocular reaction to lens capsule rupture in domestic animals
• Metaplastic lens epithelial cells express vimentin and smooth muscle actin, and they proliferate and migrate. They also produce collagen and secrete a thick basement membrane reminiscent of lens capsule
▪ Lens epithelial cells of dogs and cats appear to have a greater tendency to proliferate and migrate in response to injury than those of humans
– Metaplastic lens epithelial cells in damaged human lenses may migrate, but they seldom migrate away from the lens capsule
– In dogs and cats, metaplastic lens epithelial cells often migrate along the surface topography of the inner aspect of the globe, including:
○ The outer surface of the lens
○ The anterior and posterior surfaces of the iris
○ The surfaces of the detached retina
○ The inner choroid, within the sub-retinal space
– In cats, metaplastic lens epithelial cells may give rise to post-traumatic sarcoma
○ This neoplasm is discussed in detail later in this chapter.
Comparative Comments
A significant difference seen in the response to non-penetrating (blunt) ocular trauma in humans is a less aggressive spindle cell metaplasia and migration of lens epithelial cells after the lens capsule is ruptured.
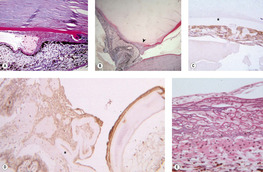 |
| Figure 5.10 Metaplasia and proliferation of released lens epithelial cells. (A) Photomicrograph of a canine globe showing the iridocorneal angle with a spindle cell proliferation and broad anterior synechia secondary to lens epithelial cell proliferation and migration into the anterior chamber. Surrounding each spindle cell is a thick PAS-positive basement membrane, characteristic of lens epithelial cell origin (PAS stain). (B) Low magnification photomicrograph showing lens capsule rupture (arrow), with proliferation and migration of metaplastic lens epithelial cells. The lens is entrapped in the anterior chamber and the proliferating cells are between the lens and the iris (PAS stain). (C,D) Photomicrographs of a traumatized dog globe showing metaplastic lens epithelial cells, labeled with immunohistochemical stain for smooth muscle actin, within the lens capsule (*) (C) and surrounding the wrinkled lens capsule (*) (D). (E) Photomicrograph of a traumatized feline globe showing the tapetum and a membrane on its inner surface composed of metaplastic lens epithelial cells. A distinct PAS-positive basement membrane surrounds individual cells (PAS stain). |
Traumatic lens subluxation or luxation
This is rarely seen in isolation, as the forces required to disrupt the zonular attachments of domestic animals are generally associated with other signs of severe ocular trauma.
Retinal effects (Figs 5.11, 5.12)
Because of the precise anatomic and functional organization of normal retinal tissue, it is at great risk of damage associated with contusive injury.
• The neuroretinal tissue is ‘stretched’ across the posterior segment, and it has a semi-liquid consistency that makes it vulnerable to disruption by the shearing forces of a pressure wave propagating within it
▪ This type of injury can be associated with ‘whiplash’ forces, which may result from shaking, as seen in the shaken-baby syndrome in human infants, or from a blow to the head
• If the damage is mild, the retina may not degenerate, and a return to function is possible
• If the damage to the retina is locally severe, the retinal tissue undergoes rapid degeneration. The damage occurs acutely, is non-progressive over the long-term, and it is often segmental
• Within the first 24 h, retinal contusion is associated with apoptosis and physical disruption
▪ Separation of the photoreceptor processes from the outer nuclear layer is seen in acute trauma
▪ Hemorrhage in the retina or vitreous
• Within 5 days, the severely affected retinal tissue becomes liquefied and phagocytes (gitter cells) predominate
• The end-stage is regional, severe retinal atrophy
▪ Retinal detachment and retinal tears are frequently seen
▪ A useful clue that trauma was involved as the cause of severe retinal damage is the finding of well-preserved blood vessels, seemingly ‘free in space’, separated from the atrophic retinal tissue adjacent to them.
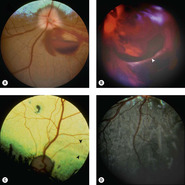 |
| Figure 5.11 Traumatic retinal tear, fundoscopy. (A) Doberman Pinscher, 3.5 years old: blunt trauma caused papilledema and preretinal hemorrhage. (B) Mixed Breed, 3 years old: extensive posterior segment hemorrhage is present after being hit by a car. The arrow points to the edge of a giant retinal tear in the detached retina. (C) Beagle, 1.5 years old: acute blindness was present after being hit by a car. Peripapillary and horizontal linear areas of tapetal hyperreflectivity are present (black arrows). Abnormal tapetal pigmentation is also present. (D) Non-tapetal fundus of the globe in (C). Linear areas of depigmentation and pigment migration in the outer layer of the retina are present. |