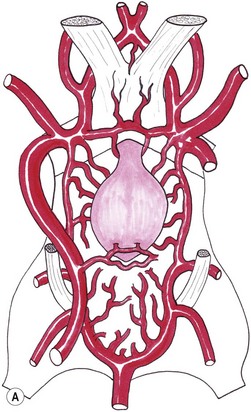
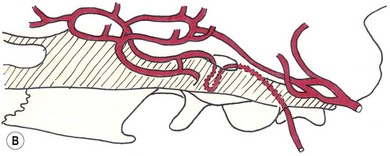
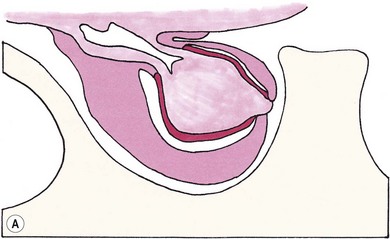
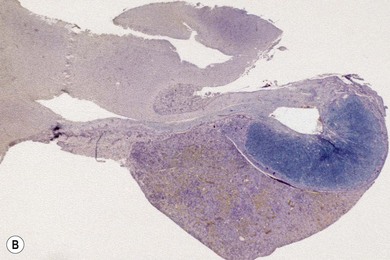
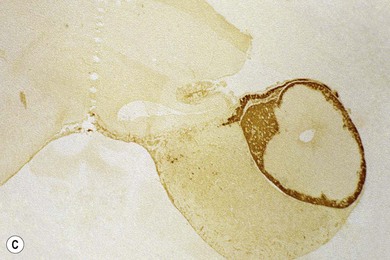
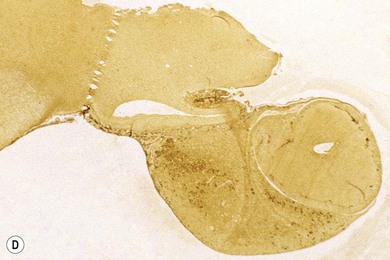
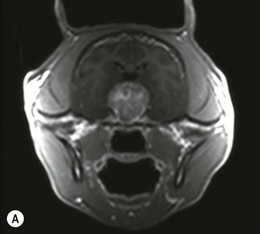
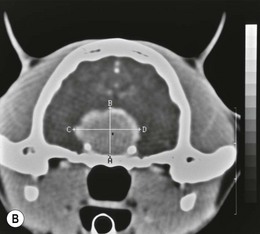
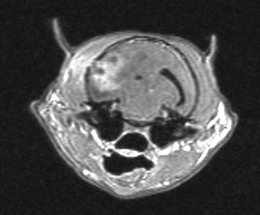
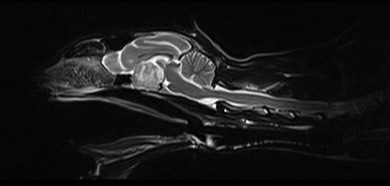
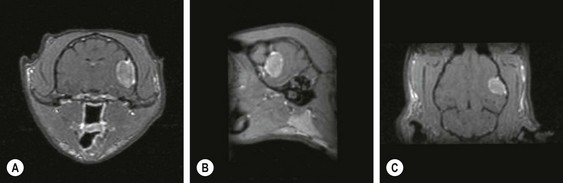
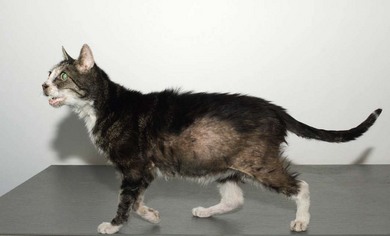
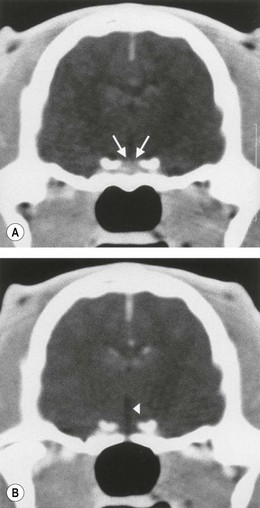
Chapter 58 The feline skull is somewhat more accessible than the canine skull due to less overlying muscle and a thinner calvarium. Also, the increased mobility of the skull in relation to the cervical spine facilitates the caudal approach because it is possible to position and stabilize the cat’s skull in hyperflexion. The function of the feline skull is to protect the brain. The brain can be divided into three large regions: cerebrum, cerebellum, and brainstem.1 The cerebrum consists of the paired cerebral hemispheres that are separated by a longitudinal fissure. The cerebral hemispheres are composed of cerebral cortex (gray matter) and underlying white matter. Each cerebral hemisphere contains a lateral ventricle that communicates with the third ventricle through an interventricular foramen. The olfactory nerve originates from the rostral extensions of the cerebrum, the olfactory bulbs. The other cranial nerves originate from the brainstem. The cerebellum is separated from the cerebrum by a transverse fissure that holds the tentorium. The brainstem occupies the middle and caudal fossae of the floor of the cranial vault and is continuous with the spinal cord. It contains the third and fourth ventricle. The thalamus, subthalamus, hypothalamus, and epithalamus are components of the brainstem. The hypothalamus is connected to the hypophysis, which resides in the pituitary fossa bordered rostrally by the tuberculum sphenoidalis and caudally by the dorsum sellae. The third ventricle runs deep into the posterior lobe (Fig. 58-1) of the pituitary gland. The pituitary gland is composed of the anterior lobe and a neurointermediate lobe. In the cat, the pars intermedia is a prominent layer, which can be visualized by immunohistochemical staining (Fig. 58-1). Figure 58-1 (A) Schematic sagittal median representation of the hypophysis in relation to the pituitary fossa. (B) PAS-Alcian Blue-orange G stain of a sagittal section of the cat pituitary. The third ventricle runs deep into the posterior lobe (blue), which is surrounded by a thin rim of pars intermedia. Sections of the cat pituitary stained with an antibody against (C) α-melanocyte stimulating hormone and (D) adrenocorticotropin, which illustrates that in the cat the anterior lobe extends upwards around the pituitary stalk. (Courtesy of Professor A. Rijnberk and Dr H.S. Kooistra.) The major venous sinuses into which the veins of the brain drain are the basilar, paired transverse, confluens, and dorsal sagittal sinuses. The locations of these sinuses are generally used as landmarks for the various surgical approaches to the brain and care should be taken to avoid them. The middle meningeal artery and its rostral and caudal branches supply the dura and the adjacent portions of the skull. The ethmoidal artery reaches the dura after passing through the ethmoidal foramen and runs between the cribriform plate and olfactory bulb. The internal carotid arteries and the basilar artery give rise through the caudal communicating arteries to the arterial cerebral circle (of Willis) at the base of the brain with the hypophysis at its center (Fig. 58-2). Arterial branches sprouting from the circle constitute the arterial supply to the brain. Rostral, middle, and caudal cerebral arteries originate from the circle and run between the gyri in the sulci. Also, the cerebellum receives blood from the circle by way of the rostral cerebellar artery. The anterior lobe of the hypophysis has no direct arterial supply but receives blood from the median eminence through long portal veins. The neurointermediate lobe receives a direct arterial supply from the fused caudal intercarotid arteries and the caudal hypophyseal artery. These run partly in the rostral intercavernous sinus and perforate the dura to connect directly to the neural lobe.2 Cats with signs attributable to a suspected intracranial mass may present in several ways and the clinical signs may represent different underlying causes (Box 58-1). The cat with an intracranial tumor is a diagnostic challenge since the mass effects can initially be absent or very subtle and clinical signs vary from mild depression to secondary epilepsy.3–5 Also, pituitary adenomas in cats manifest themselves by endocrinologic syndromes (diabetes mellitus, acromegaly, and/or Cushing’s syndrome) that may delay the diagnosis of a large intracranial pituitary mass.6 For further details on clinical signs in pituitary adenomas with endocrine syndromes, please see Chapter 35 and the sections below (Cushing’s disease and acromegaly). Historical findings in cats with intracranial tumors (other than functional pituitary adenomas) include subtle to overt altered behavior (e.g., hiding, personality changes, lethargy, poor appetite, disorientation, and aggressive behavior), seizures, impaired vision, weakness (para-, hemi- or tetraparesis), and altered consciousness (e.g., depressed, obtunded, stuporous, or comatose).7,8 The severity of clinical signs depends on the tumor location, size, invasiveness, and growth rate. For example, tumors close to the cerebellum will cause an ataxic gait whereas tumors close to the optic chiasm will impair vision. Behavioral changes (rather than seizures) are often the first and most common findings in cats with brain tumors. In a study of 61 cats with intracranial neoplasia, only 23% had a history of seizures.8 In another study of 125 cats with recurrent seizures, primary epilepsy was compared with secondary epilepsy in relation to signalment, history, ictal pattern, clinical and neurologic findings. Seizure etiology was classified as primary in 47 (38%) and secondary in 78 (62%) cats. Secondary epilepsy was caused by intracranial neoplasia in only 16 of 78 cats.9 Abnormal clinical findings associated with brain neoplasia include weight loss despite normal appetite, decreased and no appetite, decreased thirst, and bradycardia. Neurologic abnormalities include seizures, circling, locomotor disturbances, visual deficits, hearing loss, altered states of consciousness, and head pressing. Acute post-retinal blindness may be the sole neurologic deficit.10 Neurologic signs alone may be enough for neuroanatomic localization of the tumor. Characteristic neurologic signs of focal intracranial lesions affecting the forebrain (seizures), cerebral cortex (head pressing), thalamus (personality changes, visual deficits), brainstem (cranial nerve deficits), vestibular systems (nystagmus), and cerebellum (ataxia, intention tremor) may help neurolocalization, but precise pinpointing to a specific region is often difficult since neurologic examination in cats may be hampered by their unwillingness to cooperate, absence of cranial nerve deficits and overlap of neurologic signs. Skull radiography is not a valuable diagnostic tool for differentiation or localization of primary intracranial neoplasia, since mineralization and invasion into bone are rare.5 Exceptions are hyperostosis adjacent to the meningioma with invasion of the calvarium causing erosion or complete calvarial disruption,11 invasion of the calvarium and brain by local extension (e.g., with skull osteosarcomas, nasal adenocarcinomas, and squamous cell carcinomas of the middle ear), and calcification or mineralization of an intracranial neoplasm (e.g., with meningioma). MRI is an excellent diagnostic tool for detection of intracranial tumors in cats and it provides important information to aid in the diagnosis of tumor type.12,13 MRI of the feline cranium has been performed using a low-field open magnet (0.2 Tesla) or a high-field closed magnet (1.5 Tesla).12 Transverse spin echo T1-weighted, proton density-weighted, and T2-weighted images are usually acquired before contrast injection. Transverse spin echo and gradient echo T1-weighted images can be acquired after intravenous contrast injection (gadolinium dimeglumine, 0.1 mmol/kg). MRI and contrast-enhanced CT14,15 are the methods of choice for pituitary imaging as they can confirm the diagnosis of a pituitary tumor (Fig. 58-3). When pituitary surgery is a treatment option, CT is preferred to low-field MRI because of better resolution with modern CT scanners and the ability to precisely define the surgical landmarks.16 Contrast-enhanced MRI16,17 and CT18,19 reveal the pituitary dimensions (height, width, length) and allow for calculation of the pituitary height/brain area (P/B) ratio.20 However, it must be emphasized that the reference range for non-enlarged (P/B ≤0.31) versus enlarged (P/B >0.31) pituitaries was established for canines, and it is the author’s experience that this is somewhat different for felines. The upper normal P/B ratio for the cat is most likely higher and in the range of 0.40, but this still needs to be established. Dynamic CT also allows for visualization of the neurointermediate lobe (NIL), and analysis of its enhancement, the so called ‘pituitary flush’. Distortion or displacement of the flush gives insight into lateralization (left/right) or dorsal/ventral localization of the adenoma.18 Figure 58-3 (A) Transverse T1-weighted MR image in a 10-year-old male European short-hair cat with post-retinal blindness, aggressiveness and hypersensitive behavior shows a large pituitary mass with a diameter of 17 mm. (B) Contrast-enhanced transverse CT image in a 14-year-old castrated male domestic short-haired cat presented with mild clinical signs of hyperadrenocorticism and central blindness shows a very large pituitary tumor (A–B = 13.6 mm; C–D = 17.9 mm). CT is superior to MRI for imaging the tumor mass in relation to bony landmarks. Trauma to the feline calvarium occurs frequently and is mainly caused by motor vehicle accidents, penetrating bites or gunshot wounds, falls, and kicks.5 Severe trauma is generally necessary to cause significant brain injury due to the protective musculature and calvarium. More than 50% of head trauma patients have associated injuries including thoracic trauma (pulmonary edema, pneumothorax, contusion) and fractures (spine, pelvis, long bones, mandible, maxilla).5 Diagnostic imaging of head trauma patients should include thoracic radiographs to assess for thoracic abnormalities. Medical treatment should be the first line of treatment in head trauma patients and is directed at reversing brain edema, hypoxia, ischemia, and increased intracranial pressure and preventing infection. Stuporous or comatose cats presented with head trauma should be initially treated with appropriate fluid therapy to maintain systolic blood pressure. The cat should be placed in an oxygen-rich environment (e.g., oxygen cage, nasal oxygen) and efficacy of oxygen supplementation can be monitored with arterial blood gas analysis, arterial blood pressure measurements through arterial catheterization, electrocardiography, capnography, and pulsoximetry. Mannitol (20%) in a dosage of 0.5–1.5 g/kg IV over 20 minutes (limited to three boluses per 24 hours) is administered to decrease intracranial pressure. Furosemide (2.2–4.4 mg/kg IV) is synergistic in reducing intracranial pressure. Methylprednisolone sodium succinate (30 mg/kg IV, repeat after six hours) given within 12 hours after trauma is thought to help restore membrane integrity by removing free oxygen radicals from the damaged brain area.5 Seizures can be controlled with anticonvulsants (diazepam 0.5 mg/kg bolus IV, repeat if necessary; phenobarbital 2–5 mg/kg as a bolus IV, to effect). If the cat has an open wound or open skull fracture, cephalozin (20 mg/kg IV, repeat every eight hours) is started and continued orally for ten days. If the lesion is located in an area amenable to surgical decompression (i.e., through craniotomy) and is removable (e.g., hematoma and fracture fragment) then surgical intervention should be considered (see Box 58-2). Depressed skull fractures can be carefully reduced; skull fragments are removed when they are embedded in brain parenchyma. Underlying dura, arachnoid, and brain parenchyma are examined for hemorrhage. If an epidural, subdural, or subarachnoid hematoma is seen, the hematoma is gently lifted with a brain spatula and controllable suction. Small dural and arachnoid vessels are cauterized with bipolar cautery and pieces of thrombin gel foam are used to enhance coagulation. After hematoma removal the surgical field is gently irrigated with warmed sterile saline (NaCl 0.9%) solution. Dural tears are not closed and fracture fragments are not replaced. Brain abscesses are extremely rare in cats and it was only recently that the first well documented case was described, suspected to be secondary to a bite wound.21 A nine-year-old male castrated European short-hair cat was presented with a six-day history of progressive depression and ataxic gait. Neurologic examination revealed depression, absent menace in the left eye, absent pupillary light reflex in the right eye, anisocoria, circling to the right, and delayed proprioception in all limbs. MRI showed a space-occupying right temporal lobe lesion adjacent to a small defect in the temporal bone suggestive of a meningoencephalitis with concurrent abscess formation (Fig. 58-4). The site was surgically approached by a rostrotentorial craniectomy. A cerebral abscess was found and debrided. Propionibacteria spp. was cultured from the purulent fluid. Histopathologic examination of the removed tissue demonstrated a subacute to chronic purulent encephalitis with extensive necrosis of brain tissue. Neurologic signs resolved completely within two weeks and full recovery was observed four weeks after surgery. Figure 58-4 Transverse T1-weighted image in a nine-year-old male castrated European short-hair cat with a history of depression and ataxic gait. A space-occupying, contrast enhancing lesion is present in the right temporal musculature and right temporal cerebral lobe. A midline shift with compression and displacement of the right and lateral ventricle is apparent. After craniectomy and dural incision a yellow viscous discharge was found, confirming the diagnosis of cerebral abscess. The incidence of intracranial neoplasia in cats is approximately 3.5 per 100 000.6 The most common primary brain tumor is meningioma. Primary brain tumors other than meningiomas, such as gliomas (astrocytoma, oligodendroglioma), choroid plexus tumors, or ependymoma are rare in cats. Meningiomas are mesenchymal tumors that arise from the dura mater, arachnoid (most commonly), or pia mater. Feline meningiomas are generally solitary but multiple meningiomas have been reported.22–24 Meningiomas are most commonly located over the cerebral hemispheres but may also originate in other locations, e.g., rostral to the pituitary fossa in the area of the optic chiasm (Fig. 58-5). Meningiomas grow slowly, accounting for the insidious onset of clinical signs, usually starting with behavioral changes. The average age of cats with meningioma is 12 years (range five to 17 years). A gender predilection has not been identified, although some reports suggest a higher incidence in male cats. All breeds of cats appear to be affected by meningiomas. Diagnosis is confirmed with MRI: meningiomas typically show hyperintensity of the mass lesion on T1-weighted pre- and post-contrast sequences (Fig. 58-6).3 The dural tail sign seen on MRI is also very specific for meningioma.25 However, absence of hyperintensity and the dural tail sign in some cases and the overlap in the MRI appearance between different types of primary brain tumors often precludes a definite diagnosis of meningioma on the basis of imaging alone. Figure 58-5 High-field (1.5 Tesla) MRI in a 10-year-old male European short-hair cat with post-retinal blindness, aggressiveness and hypersensitive behavior. Sagittal T2-weighted gadolinium-enhanced MR image shows a large hyperintense intracranial mass in the planum sphenoidalis with extension into the pituitary fossa. Figure 58-6 (A) Transverse, (B) parasagittal, and (C) dorsal T1-post contrast (gadolinium) T1-weighted MR images in a 12-year-old cat with a history of seizures. A hyperintense extra-axial intracranial mass was found. The dural tail sign is visible on the transverse image at the upper margin of the tumor close to the skull bone (A). Surgery and histopathology confirmed the diagnosis of meningioma. Meningiomas, particularly those located over the cerebral convexities or in the frontal lobes of the cerebrum, may often be completely removed by surgery (see Box 58-2). Final diagnosis is based on histology with hematoxylin and eosin staining.13 Immunohistochemistry for vimentin, CD34, and E-cadherin is proposed to support a diagnosis of meningioma in surgical or postmortem samples.26 Studies have also shown that expression of metalloproteinase-2, progesterone receptor, and Ki67 holds some prognostic value in feline meningiomas.27,28 Surgical removal of solitary meningioma in cats results in excellent long-term survival and is the first choice of treatment. In a series of ten cats, one cat died immediately after surgery, and three cats died of concurrent disease unrelated to cerebral neoplasia two to 30 months after surgery.29 In another study, four cats that had surgical removal of solitary meningioma had a mean survival time of 485 days, with two cats surviving more than two years after surgery.30 A report of 17 cats with meningioma treated by surgical removal indicated that three died in the immediate postoperative period, three cats died with tumor recurrence at three months, nine months, and 72 months after surgery, and the remaining 11 cats survived without tumor recurrence for 18 to 47 months (median 27 months) postoperatively.31 The results of craniotomy in 42 cats with meningioma indicated a median survival of 26 months (range three days to 54 months) and the overall survival was 71% at 6 months, 66% at one year, and 50% at two years.32 In a report on surgical removal of tentorial meningiomas in six cats, one cat died immediately postoperatively and five cats survived with a median survival of 20 months (range six to 30 months) but all died or were euthanized because of tumor regrowth.33 Pituitary adenomas are considered benign tumors although in humans they have been reported to invade surrounding tissues such as the dura mater, the cavernous sinus, and the sphenoid sinus.34 Due to their extension and infiltration of regional structures, these invasive adenomas have a high rate of recurrence after surgical resection in humans. Pituitary adenomas in cats show a greater diversity in cell characteristics than their canine counterparts. In addition to corticotroph (ACTH cell) adenomas, causing pituitary-dependent hyperadrenocorticism or Cushing’s disease,2,4 and somatotroph (GH cell) adenomas causing acromegaly,35 a melanotroph (α-MSH cell) adenoma36 and double (ACTH and GH cell) adenomas37,38 have also been described in cats. Prolactin (PRL) cell adenomas (prolactinomas) have not been described in cats. Endocrinologically non-functioning pituitary tumors occur in older cats and comprise 9% of the feline intracranial neoplasia that leads to neurologic signs due to tumor mass effect on the brain.7 Cortisol is the main glucocorticoid released by the adrenal glands in cats. Thus endogenous glucocorticoid excess is essentially hyperadrenocorticism. Prolonged exposure to inappropriately elevated plasma concentrations of free cortisol leads to a variety of signs consistent with Cushing’s syndrome. In cats, pituitary-dependent hyperadrenocorticism is a rare disease of middle-aged and older animals39 and the disease is the result of excessive ACTH secretion by a pituitary adenoma. The pituitary lesions producing the excess of ACTH range from small hyperplastic cell nests of corticotroph (or melanotroph) cells to adenomas and large tumors. Pituitary adenomas may extend and infiltrate into surrounding tissues such as cavernous sinus, dura mater, brain tissue, third ventricle and rarely, sphenoid bone. These are called invasive adenomas, whereas only the exceptional tumors associated with extracranial metastasis are called carcinomas. Corticotroph adenomas may co-occur with somatotroph adenomas in the cat and these are called double adenomas.37,38 Many clinical signs can be related to the actions of excessive glucocorticoids, i.e., increased gluconeogenesis, lipogenesis and protein catabolism. The cardinal physical features are central obesity and atrophy of muscles and thinning of skin. In addition, polyuria and polydipsia and polyphagia are often dominant features. Depending on the duration of the glucocorticoid excess the changes may range from cessation of shedding, no regrowth of clipped hair, some thinning of the coat, to alopecia and a thin and wrinkled skin (Fig. 58-7A). The skin thinning and the immune suppression make the animals prone to skin lesions and skin infections (Fig. 58-8). The polyuria is known to be due to both impaired osmoregulation of vasopressin release and interference of the glucocorticoid excess with the action of vasopressin. In cats, the cutaneous manifestations are initially less pronounced than in the dog. Furthermore, glucocorticoid excess gives rise less readily to polyuria/polydipsia in cats, and it may become obvious only when diabetes mellitus develops. Figure 58-7 (A) Lateral view of a six-year-old Persian cat with pituitary-dependent hyperadrenocorticism showing alopecia and a thin and wrinkled skin with folds. The urinary corticoid/creatinine ratio was 73 × 10−6 which is above the upper limit of the reference range (42 × 10−6). (B) At four months after trans-sphenoidal hypophysectomy the hair coat had regrown on the abdomen and flank of the cat and the UCCR was 1.3 × 10−6. Figure 58-8 A 17-year-old castrated male cat, referred because of problems controlling its diabetes mellitus. In addition to polyuria, polydispia, and weight loss, there was alopecia and muscular weakness in the hind legs. Basal UCCRs on two consecutive days (73 × 10−6 and 88 × 10−6) were above the upper limit of the reference range (42 × 10−6). After three oral doses of 0.1 mg dexamethasone per kg body weight the UCCR decreased to 9 × 10−6 indicating pituitary-dependent hyperadrenocorticism. CT revealed the pituitary to be moderately enlarged (height 4.2 mm, width 4.0 mm) with displacement of the neurohypophyseal flush. Cats are susceptible to the diabetogenic effects of glucocorticoids. In the majority of the described cases of feline hyperadrenocorticism the disease was associated with diabetes mellitus and the suspicion of hyperadrenocorticism has often arisen specifically because of insulin resistance encountered in the treatment of diabetes mellitus.39 Endogenous glucocorticoid excess increases blood pressure. In cats with hyperadrenocorticism decreased thyroxine (T4) concentrations can be found, as a consequence of altered transport, distribution, and metabolism of thyroxine, rather than hyposecretion. Clinical neurologic manifestations of the disease are only observed when the pituitary tumor develops to be large enough to cause a mass effect.40 These neurologic abnormalities are often vague, including lethargy, inappetence, and mental dullness. The tests for diagnosis of hyperadrenocorticism are described in Chapter 35. Resistance to glucocorticoid feedback is significantly correlated with the size of the pituitary gland. Large tumors tend to be more resistant to the suppressive effect of dexamethasone due to their size, and they often release ACTH precursors like pro-opiomelanocortin (POMC). There is also a report of a cat with a melanotroph pars intermedia adenoma with extremely elevated plasma concentrations of α-MSH and no evidence for ACTH-dependent hyperadrenocorticism.36 Diagnostic imaging may help to complete the picture of the physical and hormonal changes that can be associated with hyperadrenocorticism (see Chapter 35).35 Ultrasonography of the adrenal glands and CT or MRI of the pituitary (see above) and adrenal glands are the imaging techniques now most frequently used, especially in the search for the location and the characterization of the source of the hormone excess. This visualization is imperative in institutions where hypophysectomy or pituitary irradiation is an option for treatment. For hypophysectomy the surgical landmarks in relation to the pituitary tumor are best visualized with contrast-enhanced CT (Fig. 58-9) and for pituitary irradiation using a linear accelerator the exact pituitary zones for intense radiation need to be outlined with MRI or CT. Figure 58-9 (A) Contrast-enhanced transverse CT images through the pituitary fossa in a seven-year-old European short-hair cat with Cushing’s disease. The urinary corticoid/creatinine ratio was 77 × 10−6 (reference range 8 × 10−6 to 42 × 10−6). The pituitary (arrows) is not enlarged and is 3.5 mm high, 4.7 mm wide, and the pituitary height/brain area ratio is 0.36. (B) At three months after trans-sphenoidal hypophysectomy, the UCCR was 2.3 × 10−6 and there was complete remission of signs of hyperadrenocorticism. There is no contrast enhancement in the pituitary fossa, and the third ventricle is indicated (arrowhead). Spontaneous recovery of pituitary-dependent hyperadrenocorticism is rare and life expectancy in severe cases is usually less than one year if the disease is left untreated. Death may ensue as a result of complications such as heart failure, thromboembolism, or diabetes mellitus. In mild cases with apparently little progression, the course of the disease may be followed for some time with measurements of urine cortisol/creatinine ratio (UCCR), although the usefulness of UCCR is debatable due to a high rate of false positives. To minimize the risk for false positives repeated urine samples are needed that are collected in a stress-free environment. The treatment of pituitary-dependent hyperadrenocorticism should ideally be directed at the elimination of the stimulus for the augmented production of cortisol, i.e., the pituitary lesion causing the excessive ACTH secretion. In the last decade experience has been gained with a microsurgical technique for transsphenoidal hypophysectomy (see Box 58-3) in the treatment of cats with pituitary-dependent hyperadrenocorticism.41 With appropriate short-term and long-term substitution therapies, pituitary surgery is an effective treatment. It can only be performed in specialized institutions with intensive perioperative care and where imaging techniques such as CT and MRI enable assessment of localization and size of the pituitary gland prior to surgery. In a study of seven cats that underwent hypophysectomy for Cushing’s disease, two cats died within four weeks after surgery of non-related disease. In the five remaining cats, hyperadrenocorticism went into clinical and biochemical remission (follow-up periods six to 46 months). Hyperadrenocorticism recurred after 19 months in one cat. In two of four cats that had concurrent diabetes mellitus, insulin treatment could be discontinued at one and five months after hypophysectomy.41 Prognosis after hypophysectomy for Cushing’s disease in cats is determined by co-existing disease, and thorough presurgical screening is imperative. The main advantage for long-term survival, compared to approaches at the adrenal level, is in the prevention of neurologic problems that may occur as a result of an expanding pituitary tumor. Radiation therapy for pituitary macroadenomas may lead to some decrease in the tumor mass and peritumoral edema, but concurrent adrenocortical suppression treatment is needed. Radiation therapy does not usually reduce the hypersecretion of ACTH sufficiently so additional therapy at the adrenal level is required.42–47 Treatment of adrenal-dependent disease is described in Chapter 35 and in the literature.42–47
Neurocranium
Surgical anatomy
The skull and brain
Vascular supply
General considerations
Brain and the mass effect
Clinical signs
Diagnostic procedures
Radiography
Advanced imaging
Surgical diseases
Trauma
Brain abscess
Neoplasia
Meningiomas
Pituitary gland adenomas
Cushing’s disease


![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Neurocranium