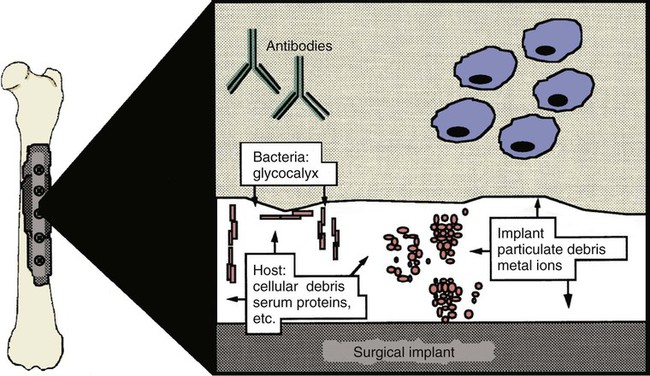
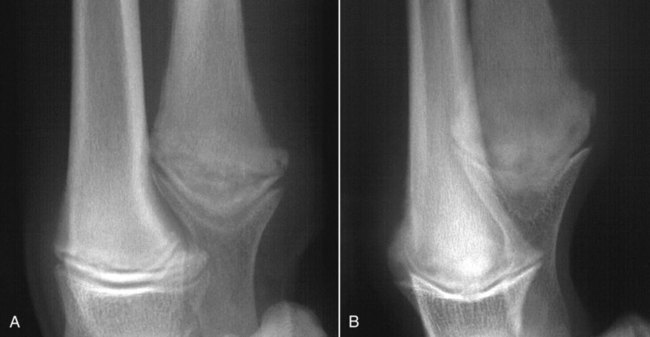
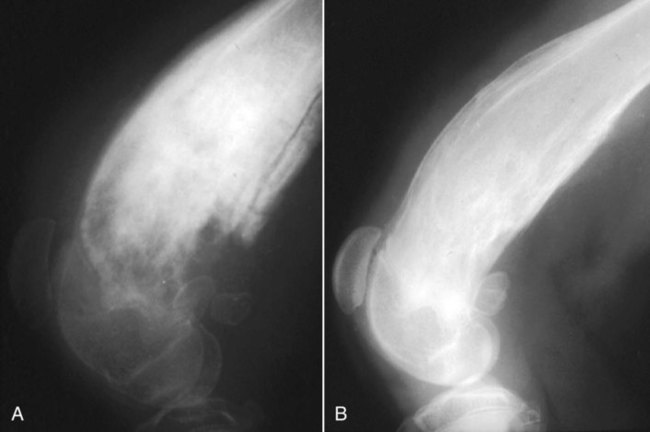
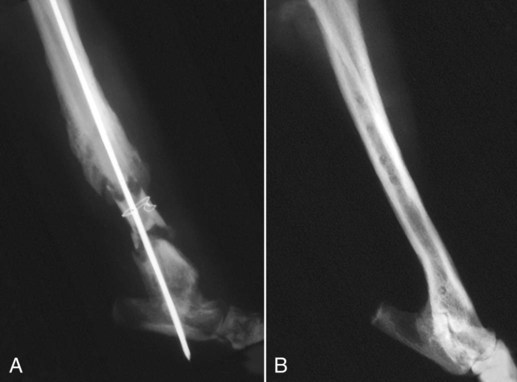
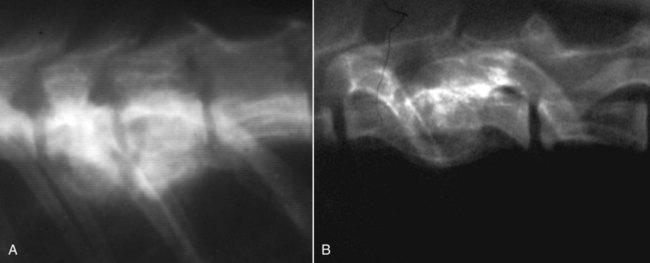
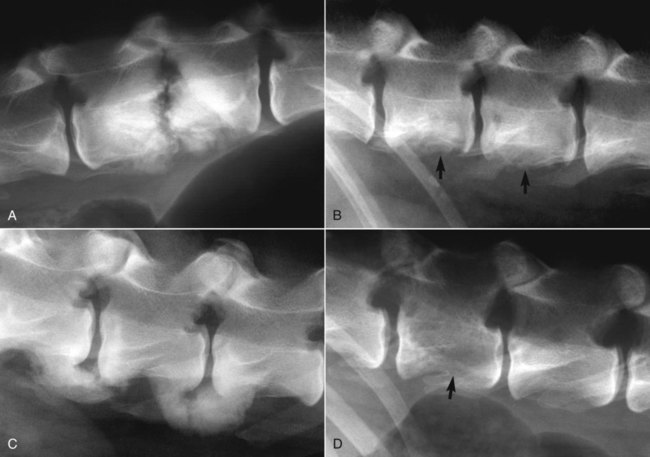
Musculoskeletal infections involve bones, joints, and muscles. Osteomyelitis is classically defined as inflammation of the cortical bone, medullary cavity, and periosteum.25,81 Most cases involve infectious agents, including bacteria, fungi, and viruses. Other potential causes include irritants, such as radiation therapy, or surgical implants; however, these causes are much less common.25,81,81 Bone infections can involve both the appendicular and axial skeletons. The appendicular infections occur in the four limbs. Axial infections can involve the intervertebral disks or vertebral bodies. Diskospondylitis with secondary osteomyelitis developing in the opposing vertebrae is more common in dogs and cats compared with humans. In humans, vertebral osteomyelitis generally occurs without disk space infection. In animals, vertebral osteomyelitis usually arises from penetrating injuries (see Actinomycosis, Chapter 47) rather than from bloodborne infections which are more often associated with diskospondylitis (see Diskospondylitis and Vertebral Osteomyelitis later in this chapter). Osteomyelitis is generally classified into hematogenous and posttraumatic types, although there is evidence that the source of infection in some cases of posttraumatic osteomyelitis is also hematogenous in origin.118 Posttraumatic osteomyelitis can be either acute or chronic.25,81,81 Because of differences in causes, clinical findings, and treatments, appendicular osteomyelitis and diskospondylitis are discussed separately (Box 85-1). In addition, otitis media is included in this chapter because the bony structures of the middle and inner ear are involved in the disease process. Joint and muscle infections that involve soft tissues adjacent to bone are each considered separately. Table 85-1 lists the microorganisms commonly associated with musculoskeletal infections. TABLE 85-1 Microorganisms Associated with Musculoskeletal Infections Craig E. Greene and David Bennett Bacterial infections cause most cases of osteomyelitis in clinical practice. In some studies evaluating the composition of involved aerobic or microaerophilic bacteria, gram-positive species such as Staphylococcus account for 50% to 60% of cases,81,121 and Staphylococcus pseudintermedius is the most common pathogen. Other gram-positive organisms are found less frequently and include Streptococcus and Enterococcus. In other reports, both gram-positive and gram-negative organisms have been isolated at approximately the same rate.60 Gram-negative organisms include Pasteurella, Escherichia coli, Pseudomonas, Proteus, Serratia, and Klebsiella. Anaerobic bacteria include Peptostreptococcus, Bacteroides, Fusobacterium, Actinomyces, Nocardia, and Clostridium.146 Anaerobic bacteria have been isolated with greater frequency, as sample collection, transportation, and incubation techniques have improved. Results of one study were anaerobes isolated from 64% of osteomyelitis cases in dogs.121,146 Anaerobic bacteria are rarely isolated alone, and other anaerobes and the aforementioned microaerophilic or aerobic bacteria usually make the environment conducive for anaerobic growth (approximately 35% of posttraumatic cases have mixed infections). Polymicrobial infections are being more commonly identified, possibly because of the misuse of antimicrobial agents, which potentially allow resistant bacterial populations to flourish, or because of better bacterial collection and detection methods. Muir and Johnson146 reported that 19 of 28 cases of osteomyelitis in dogs and cats had mixed aerobic and anaerobic infections. In human medicine, a poorer prognosis is associated with cases of osteomyelitis when more than one organism is isolated. A variety of fungi has been identified in osteomyelitis from regions where these organisms are endemic. In the United States, the most common isolates are Blastomyces, Coccidioides, Histoplasma, and Cryptococcus.105,217 Other organisms include Aspergillus and Candida. German shepherd dogs have the highest prevalence rate for hematogenous fungal osteomyelitis caused by Aspergillus, making an hereditary immune deficiency a likely contributing factor (see also Disseminated Aspergillosis, Chapter 62). Although viral agents have been incriminated as causing inflammatory bone diseases, factual data is limited. However, virulent and vaccine-strain canine distemper virus (CDV) has been suspected as a cause of metaphyseal osteopathy (MO, also called hypertrophic osteodystrophy) and juvenile cellulitis in dogs (see Chapters 3 and 100).125,135,135 Both clinical and experimental studies have shown that CDV can localize to metaphyseal bone after systemic infection and in some cases be associated with overt bone pathology.9,135 One of the authors (DB) has observed several cases of MO in Weimaraner puppies complicated by immune-mediated polyarthritis, suggesting the possibility of an underlying immunological dysfunction. One study that compared the risk factors involved with MO and CDV infection in dogs did not support a possible relationship between MO and distemper virus.147 Spread of infection via the bloodstream from a distant site is thought to be rare in dogs and cats. Immature animals are usually affected.89,105 The metaphysis is predisposed to hematogenous embolization, and this is likely to be a result, in part, of the rich blood supply and the microvascular architecture of this region in growing bone. Capillaries that extend toward the growth plate have both variable continuous and discontinuous epithelia.78,89 Terminally growing capillary buds lack a basement membrane and have discontinuous endothelium.78,89 Discontinuities allow circulating microorganisms to escape into the extravascular tissue space during a bacteremic phase. Blood flow through these capillary beds is slow, creating an ideal environment for bacterial lodgment and proliferation. Furthermore, in contrast to secondary spongiosa, leukocytes appear to be absent around primary spongiosa.78 Bacterial invasion in the region of developing bone may be opposed by only tissue-based macrophages. Unrestricted infection in the metaphyses may spread to the epiphyses, periosteum, soft tissues, and adjacent joints. In dogs and cats, transphyseal vessels are absent at birth; thus infection is usually restricted to the metaphyseal side of the growth plate.66 Normal bone is resistant to infection. Osteomyelitis is unlikely to develop in the absence of predisposing factors, which include ischemia, bacterial contamination, bone necrosis and sequestration, fracture instability, foreign material implantation, and a systemic or local alteration in immune response or tissue metabolism.25,81,95,105 Tissue trauma and subsequent vascular compromise are important factors when discussing posttraumatic osteomyelitis. Soft tissues provide the first blood supply to the ischemic bone during the initial phases of healing. Inoculation of bacteria can occur from direct penetration of a missile or other foreign body, a bite wound, an exposure of the bone via an open fracture, or a surgical intervention. However, it is also possible that a hematogenous spread of infection from a remote site to the traumatized site can explain some bone infections, the damaged tissues and inserted implants favoring the localization and colonization of bacteria.118 Avascular bone fragments provide an ideal ecologic niche for bacteria to colonize and proliferate. Fracture instability perpetuates the persistence of infection in the bone. Instability may occur when initial stabilization is inadequate or when initial fixation fails. In either case, disruption of the blood supply is caused by damage to proliferating capillaries, promoting tissue and bone necroses and bacterial colonization and growth. Implantation of foreign material has been associated with increased infection rates.73,167,167 Staphylococci are the predominant bacterial contaminants in these infections. The primary mechanism in biomaterial-centered sepsis is microbial colonization of these materials and adjacent damaged tissues.95 Tissue necrosis and inflammation along the biomaterial’s surface, associated with failure of implant integration into host tissues, serve as a glycoprotein-conditioned substratum for which bacteria have specific receptors.95 Adherent bacteria produce a matrix of condensed exopolysaccharides known as a glycocalyx.133 The biofilm is composed of a cluster of microorganisms and glycocalyx on an inanimate surface. Embedded in the biofilm mixture of glycocalyx, host-derived serum proteins, and cellular debris, bacteria often form into microcolonies (Fig. 85-1). Within biofilms, bacteria are protected from antibodies, phagocytes, and even antibacterials.95,105,105 Bacteria within biofilms have been shown to intercommunicate via cytokines,54 and this appears to assist them in adapting as a colony within their biologic environment and avoiding host defense mechanisms. The glycocalyx retards the penetration of drugs, organisms become dormant within the biofilm, and the microenvironment within adversely affects antimicrobial activity.67 Furthermore, several different types of bacteria can both coexist and replicate in glycocalyx-enclosed microcolonies resulting in mixed infections. Clinical signs of osteomyelitis vary with the type and duration of disease. Acute osteomyelitis, either hematogenous or posttraumatic in origin, produces localized pain, erythema, and soft tissue swelling. The animal is usually febrile. Elevated leukocyte counts are common. Signs of systemic illness include lethargy and inappetence.25,81,81 Chronic posttraumatic osteomyelitis is a localized disease rarely with systemic manifestations; there is generally a history of trauma or surgery with subsequent draining tracts and lameness. Nonhealing wounds that continue to drain should always be evaluated by radiography for underlying osteomyelitis. Osteomyelitis associated with fractures is most often seen in the radius/ulna (41.5% of cases) and femur (28.5% of cases).14 In young animals, hematogenous bacterial osteomyelitis often results in fever, lameness, and swelling of the metaphyseal region of long bones. Synonyms for this condition are metaphyseal bacterial osteomyelitis or bacterial metaphysitis. It has been reported in both dogs and cats.21,206 Gram-positive bacteria such as Staphylococcus are often isolated. Swelling and lameness localized to the metaphyses in a young puppy, and associated with metaphyseal bacterial osteomyelitis, can be confused with MO, which is regarded as a form of osteomyelitis, but where bacteria are not found; it is possibly a viral osteomyelitis (see earlier discussion). MO invariably affects several metaphyses in a bilaterally symmetrical fashion, but this can also be true of bacterial metaphysitis. Radiographically, the two diseases can be similar (Fig. 85-2). There may be radiolucent areas within the metaphysis (often as a radiolucent band parallel to the physis in MO), with areas of sclerosis and periosteal new bone. If several animals in a litter develop this condition, then an underlying immunodeficiency or a congenital/early neonatal infection should be considered.200 History, physical examination, and radiography will localize the site of the lesion. Nonspecific laboratory findings may include leukocytosis, increased serum concentrations of calcium and phosphorus, and high alkaline phosphatase activity. Eosinophilia, presumably due to chronic inflammation, has also been reported in one dog.59 In the acute case, the only radiographic finding may be soft tissue swelling. As the infection progresses, radiographic changes include, but are not limited to, periosteal bone proliferation, bone resorption, and increased bone density. In chronic cases, radiography can also demonstrate the presence of loose or broken implants or nonviable bone (sequestra). Unfortunately, radiography has only a sensitivity of 62.5% and specificity of 57% in the detection of osteomyelitis.20 Ultrasound has been used to detect radiolucent foreign bodies, such as grass awns, in adjacent soft tissues or within abscess cavities.91 Nuclear scintigraphy can be helpful in diagnosing osteomyelitis; technetium-99m-methylene diphosphonate, indium-111 leukocyte, and IgG scintigraphy are radionuclide imaging techniques that can provide additional diagnostic information if radiographic findings are equivocal.117,190 However, these tests require the specialized equipment of referral centers and are thus not often used. Cytologic evaluation of exudates is informative, whereas microbiologic culture of an infectious agent is definitive. Specimens from a sterile aspirate of the lesion through intact skin or from sequestra, local necrotic tissue, or implant at the time of debridement provide the most valuable material for culture and cytologic examination. For aspiration, an area of intact, unaffected skin is selected and aseptically prepared. A 20-gauge needle is then inserted to the level of the bone, followed by aspiration into a 10-mL syringe. Organisms isolated from swabs of discharges from draining tracts may be contaminants and thus not helpful in antibacterial susceptibility testing. Proper collection and transport of both aerobic and anaerobic samples is also imperative (see Chapter 29). In cases of suspected hematogenous osteomyelitis, particularly if the animal is febrile, blood should be cultured, in addition to culturing specimens from the affected bone. Anaerobic infections should be suspected when affected tissue is characterized by a foul odor, sequestra, or the presence of multiple bacterial species suggested by impression smears or histologic examination of tissue samples (see Chapter 39).146 Further suspicion of an anaerobic infection is justified if no organism is cultured from material in which cytologic findings or other clinical features indicate infection. Multifocal sterile pyogranulomatous osteomyelitis has been reported in dogs33 but probably is caused by immune dysregulation rather than infection. Animals with hematogenous osteomyelitis are often systemically ill. The infections frequently involve the metaphyseal region of the bone and do not extend into the adjacent joint (Fig. 85-3, A). However, synoviocentesis and radiography are strongly recommended to confirm this. Blood culture results may also be informative. If joints are affected, aggressive management should be undertaken as described later for monoarthropathies. As mentioned previously, an immune-mediated polyarthritis can complicate MO in some puppies. Any fluctuant, warm, or painful area around the affected bone can be aspirated for culture and antibacterial susceptibility testing. If an obvious fluctuant area is present, attempts should be made to open and copiously lavage the region with a sterile isotonic solution such as saline and to perform debridement. Systemic intravenous antibacterials should be given for a minimum of 3 to 5 days before changing to the oral route. Oral administration should continue for a minimum of 21 days, and the choice of antibacterial should be based on culture and susceptibility testing. If the susceptibility results are not available, bactericidal agents that are active against β-lactamase-producing Staphylococcus should be used (Tables 85-2 and 85-3). Specific treatment for bacteremia or septicemia should be given if necessary (see Chapters 36 and 86). Radiographs taken 2 to 3 weeks after treatment can help evaluate progression of the lesions and response to therapy (see Fig. 85-3, B). Systemic manifestations and presence of joint involvement indicate a worse prognosis.89 TABLE 85-2 Recommended Therapy for Musculoskeletal Infections in Dogs and Cats A, Arthritis; D, diskospondylitis; I, otitis media/interna; O, osteomyelitis; M, myositis. aDrugs are listed in order of most desirable choice first. See Chapter 30 and the Drug Formulary in the Appendix. bUse minocycline or doxycycline. cSubstitute amoxicillin, first-generation cephalosporin, or erythromycin. dSubstitute any first-generation cephalosporin. eSubstitute any β-lactam-resistant penicillin. TABLE 85-3 Drug Dosages for Treatment of Musculoskeletal Infections B, Both dog and cat; C, cat; D, dog; IM, intramuscular; IV, intravenous; PO, by mouth SC, subcutaneous. bDose until a total of 8–12 mg/kg (dog) or 4–8 mg/kg (cat) is reached. aDuration of therapy depends on the tissue, site, and duration of infection; see text for guidelines. For additional information and dosages on drugs listed here, refer to the Drug Formulary in the Appendix. The prevalence of osteomyelitis is increased in open fractures when bacteria have the ability to invade the wound directly from the environment. However, most cases of posttraumatic osteomyelitis are acquired in the hospital environment and involve staphylococci or enteric gram-negative organisms such as Pseudomonas. Osteomyelitis developing within 2 to 5 days after an insult may be difficult to differentiate from a simple soft tissue wound infection. Regardless, treatment is similar, and absolute differentiation is not always possible or necessary. Treatment should be aggressive in an effort to prevent these acute infections from developing into chronic sequestered foci of infection. Management includes drainage and debridement, systemic antimicrobial agents, rigid stabilization, direct bone culture, and delayed wound closure (Fig. 85-4, A).25,81,81 Meticulous drainage and debridement of all necrotic tissues including bone, hematomas, and abscesses should be done first and is an essential component of treatment. Culture and biopsy samples are obtained at this time, followed by copious lavage of the area with lactated Ringer’s solution or saline. Drainage using closed suction units and open-wound management with daily flushing are options to be considered. Initial antimicrobial therapy is similar to that of hematogenous osteomyelitis; the most common bacterium is β-lactamase-producing Staphylococcus (see Tables 85-2 and 85-3). Drugs should be given parenterally (preferably intravenously) for the first 3 to 5 days followed by oral therapy for a minimum of 4 weeks; many cases require 8 weeks of therapy. Therapeutic agents may be adjusted as culture results become available. Patient monitoring must be intense and regular. In all cases, radiographs should be taken 2 to 3 weeks after intervention and then sequentially as needed (see Fig. 85-4, B). Chronic posttraumatic osteomyelitis is the most common type seen in veterinary practice. Because of devitalized tissues, therapy with antibacterials alone is usually not successful. Drugs cannot enter the tissue in which they are most needed. Without improving the ischemic, necrotic environment, success in bacterial eradication is minimal. Treatment is based on the same fundamental objectives of debridement of necrotic tissue and removal of bony sequestra and all foreign material, including, when appropriate, surgical implants. Attempted bacterial isolation, obliteration of dead space, establishment of drainage, and rigid stabilization of bone should be carried out. Bone will actually heal in the presence of infection if it can be stabilized. Use of external skeletal fixation, in conjunction with open wound management, has proven successful in resolving some infected fractures in dogs.148 Antimicrobial therapy must be based on culture and susceptibility results; however, correlation of clinical response with the apparent in vitro susceptibility of some microorganisms is often lacking. This may be attributed in part to the inability of antibacterials to achieve sufficient concentrations in affected tissues. With this limitation, the best chance to achieve and maintain antibacterial drug levels at therapeutic concentrations would be intravenous infusions over a minimum treatment period of 4 to 6 weeks. Unfortunately, in practice, financial constraints usually limit treatment to short parenteral regimens followed by orally administered antimicrobials. Antibacterials that can be used orally for long-term treatment and that are effective against β-lactamase-producing Staphylococcus should be considered (see Tables 85-2 and 85-3). Placement of local infusion drug delivery systems is receiving a great deal of research interest. Use of implants impregnated with antibacterials is common in human medicine, less frequent in veterinary medicine. Carriers are usually either a form of bone cement or a biodegradable polymer.27,205,205 Polymethylmethacrylate (PMMA) is an implant material that has been used successfully with numerous antibacterial agents, including vancomycin, clindamycin, tobramycin, and gentamicin. Once elution of the antibacterial agent is complete, bacterial biofilms can theoretically colonize the foreign material if it is not biodegradable (like PMMA). Treatment of severe orthopedic infections in humans and animals has included the use of antimicrobial-impregnated beads at the site of infection. Slow release in the wound site allows for high concentrations of antimicrobials in the tissue for prolonged periods. Levels of up to 200 times that after systemic administration can be achieved for as long as 80 days after implantation.216 Aminoglycosides have been the most commonly used antibacterial agents used in this way. Despite the high concentrations at the wound site, serum and urine concentrations of antibacterials do not reach toxic levels. In an experimental study in dogs evaluating PMMA bead implantation to treat osteomyelitis, there was radiographic, bacteriologic, and pathologic evidence of efficacy 6 months after implantation.210 PMMA is less than ideal in that it is not degraded and can result in a foreign body reaction, or by itself can act as a focus for bacterial colonization. Use of newer biodegradable polymers has resulted in fewer side effects. Polyglycolic beads containing gentamicin have effectively controlled staphylococcal osteomyelitis in dogs.86 Similar antibacterial-impregnated polymers have also been used to coat metallic implants. Other polymers such as bioerodible polyanhydrides and more natural substances such as hydroxyapatite or collagen have been impregnated with antibacterial agents and have shown some early success. Whenever a patient suffers a fracture complication with delayed healing, particularly if infection has occurred, surgical implants should be removed once the fracture has healed. This will help reduce the chance of a fracture-associated sarcoma developing sometime in the future.12 Prophylactic antimicrobial therapy is an accepted management for dogs and cats undergoing prolonged surgical procedures and for those animals with an increased risk of postoperative infection. Dogs undergoing elective orthopedic surgery had a reduced rate of infection compared with saline-treated control dogs when they were given prophylactic penicillin G or cefazolin.215 The choice of antimicrobial therapy should be based on the type and location of the surgical procedure and the most likely organisms to be involved in secondary contamination. Antimicrobials are administered either 30 minutes before or during the operative period at an interval of every 90 minutes and are not continued beyond the operative period. For further information, see also prophylactic antibacterial therapy in Chapter 30, Nosocomial Infections in Chapter 93, and Bacteremia and Dental Disease in Chapter 88. Craig E. Greene and David Bennett Diskospondylitis is defined as inflammation of an intervertebral disk and adjacent vertebral end plates and bodies. Infections localized to the vertebral body are usually caused by penetrating wounds, paravertebral infections, or foreign bodies; such infections are less frequent in dogs than cats. Vertebral osteomyelitis, with or without disk involvement, often involves the adjacent soft tissues and meninges. The most common cause of these processes is bacterial infection; however, fungal infections have also been reported (see Table 85-1).141 Any vertebral space can be affected; the thoracic and lumbar sites are the most commonly involved. S. pseudintermedius is the most common causative bacterium identified in the dog.28,112,113,141 Other frequently documented bacterial pathogens include Streptococcus, Brucella canis, and E. coli.28,112,113,141 Less common isolates have been Pasteurella spp., Proteus spp., Corynebacterium spp., Actinomyces, Nocardia spp., Bacteroides spp., Mycobacterium spp., Pseudomonas aeruginosa, Enterococcus faecalis, Bordetella spp., and Staphylococcus epidermidis.1,18,18 The wide variety of bacterial species underscores the need to at least attempt to obtain bacterial isolation and antibacterial susceptibility testing in every case. Fungi are involved in some infections, and most common genera have included Aspergillus, Fusarium, and Paecilomyces.30,80,112,141 In addition to the hematogenous localization of microbial organisms, migrating foreign bodies have resulted in vertebral body osteomyelitis (see Actinomycosis, Chapter 47). Plant materials have been the most widely associated foreign bodies, and geographic regional differences influence the specific causative agent.141 Postsurgical diskospondylitis may be caused by direct inoculation during a diagnostic or operative procedure on the vertebral column or by spread from other infected foci to the intervertebral site. Injury to intervertebral endplates or operative trauma to small vessels causing tissue necrosis or hematoma formation provides an ideal environment for bacterial growth.104 Epidural abscess formation and diskospondylitis can develop after epidural injections.174 Spinal column and disk infections have also been sequelae of spinal surgery, including disk fenestration. Few reports of diskospondylitis in the cat have been published.5,126,149,154,211 In all of these cases, soft tissue injury from trauma or fighting (e.g., cat bites) has been the underlying cause. Because these are spreading infections from adjacent soft tissues, they are more appropriately regarded as cases of primary vertebral osteomyelitis with secondary disk involvement. Compared with disk infection in dogs, meningomyelitis has been more commonly associated with these infections in cats. E. coli was isolated from the cerebrospinal fluid of one cat.5 One of the authors (CEG) has also documented diskospondylitis in a 6-month-old old purebred cat caused by bacteremia with a group G streptococcus presumably acquired as a neonate (see Chapter 33). In this cat, the intervertebral disk space was affected and the lesion progressed to markedly involve the adjacent vertebral bodies (Fig. 85-5). Diskospondylitis in dogs is thought to arise from the hematogenous spread of the organism into the disk space and subsequently into the adjacent vertebrae. The most commonly incriminated sources include urogenital tract and skin infections, dental disease, and valvular endocarditis, all of which may not be clinically evident. However, in many cases, no primary source of infection is apparent. Preferential hematogenous localization to the disk space probably occurs as a result of retrograde blood flow into the vertebral sinuses or because of the subchondral vascular loops in the vertebral epiphysis that slow blood flow.112,113,113 Predisposing factors have included immunosuppression and previous trauma, including surgical intervention (see Box 85-1).112,113 Clinical presentation can vary; however, in general, signs progress slowly. Although any dog or cat is susceptible to this disease, the most common signalment is that of a young to middle-aged, male, large-breed dog,112,113 although other studies have shown a higher risk in older (more than 5 years) male purebred dogs.28 Signs can vary from those of systemic illness (depression, anorexia, fever, weight loss) to musculoskeletal dysfunction. Signs of paraspinal hyperesthesia are the most characteristic of this disease and include abnormal gait and reluctance to rise or ambulate. Neurologic signs, which often develop subsequent to hyperesthesia, are those of extradural compression, resulting in paresis and ataxia, or paralysis (Fig. 85-6). The neurologic deficits depend on the site and severity of the vertebral lesion. Paraspinal hyperesthesia is often noticed first, although some stoic dogs do not show overt signs of discomfort. Neurologic dysfunction, demonstrated by paresis, can develop gradually, although sudden onset of paraplegia can occur with intervertebral disk rupture from weakened ligaments and annulus. Monoparesis or monoparalysis can result from nerve-root involvement due to bony proliferation. The most commonly reported sites of disk infection are L7-S1, midthoracic, and caudal cervical28,112,112; multiple disks can be affected in a single patient. A tentative diagnosis is made from the patient history and the general physical and neurologic examinations. Hyperglobulinemia, associated with the chronic antigenic stimulation from the persisting bacterial infection, may be apparent on blood examination. A definitive diagnosis is generally made from the spinal radiographs. Changes include concentric lysis of adjacent vertebral endplates giving an apparently increased disk space, vertebral body osteolysis or proliferative sclerosis, vertebral body shortening, narrowing and irregularity of disk spaces, and ventral osseous bridging (Fig. 85-7, A). The last must be differentiated from other vertebral lesions, including primary vertebral osteomyelitis (see Fig. 85-7, B), spondylosis deformans (see Fig. 85-7, C), and neoplasia (see Fig. 85-7, D). The earliest sign is often a reduced disk space, and this can be very subtle and difficult to appreciate; this is presumably the result of intervertebral disk cartilage destruction before bony involvement occurs. Extensive vertebral body infection may result in vertebral body collapse or subluxation.23 Radiographic changes associated with diskospondylitis can take 2 to 4 weeks to develop,113 and thus sequential radiographic evaluation may be needed to confirm the diagnosis in an animal with initial signs of hyperesthesia. Clinical signs and radiographic severity of lesions do not always correlate. In some cases, soft tissue opacities may be apparent ventral to the intervertebral spaces as assessed by radiography, ultrasonography, or other imaging methods. Computed tomography (CT) and magnetic resonance imaging (MRI) have added a new dimension to the detection of infective lesions of the spine. These more sensitive techniques are always indicated when clinical suspicion is high but spinal radiography is inconclusive. CT can also be used to guide fine-needle aspiration and tissue-core biopsy.208 Early MRI changes are decreased intensity and definition on T1-weighted images and increased intensity of the disk and endplate on T2-weighted images. Later, destruction of the vertebral endplate occurs. With T1-weighted scans after gadolinium-diethylenetriamine pentetic acid, enhancement occurs between the disk and endplate. Scintigraphy has also been used and may show increased uptake of radiopharmaceuticals at the affected disk space and endplates.190 Technetium-99m-methylene diphosphonate, gallium-67, and indium-111-labeled leukocytes may become more routine in veterinary referral centers to augment the diagnosis of occult diskospondylitis.190 In humans and dogs, tissue-core biopsy has been superior to fine-needle aspiration for obtaining positive culture results.208 For aspiration, which is often done with fluoroscopy, 0.5 mL of sterile saline is injected into the disk space and immediately aspirated to retrieve a specimen.
Musculoskeletal Infections
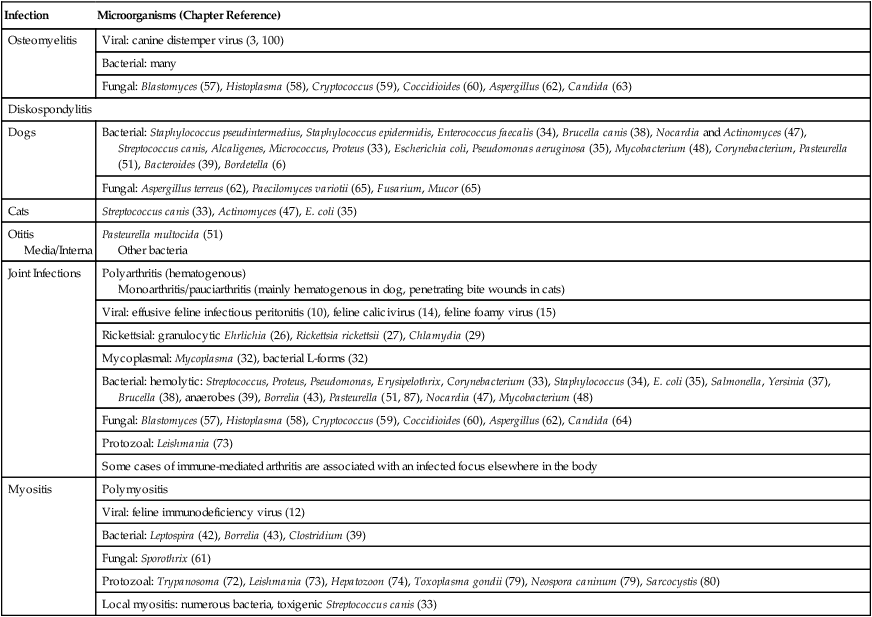
Infection
Microorganisms (Chapter Reference)
Osteomyelitis
Viral: canine distemper virus (3, 100)
Bacterial: many
Fungal: Blastomyces (57), Histoplasma (58), Cryptococcus (59), Coccidioides (60), Aspergillus (62), Candida (63)
Diskospondylitis
Dogs
Bacterial: Staphylococcus pseudintermedius, Staphylococcus epidermidis, Enterococcus faecalis (34), Brucella canis (38), Nocardia and Actinomyces (47), Streptococcus canis, Alcaligenes, Micrococcus, Proteus (33), Escherichia coli, Pseudomonas aeruginosa (35), Mycobacterium (48), Corynebacterium, Pasteurella (51), Bacteroides (39), Bordetella (6)
Fungal: Aspergillus terreus (62), Paecilomyces variotii (65), Fusarium, Mucor (65)
Cats
Streptococcus canis (33), Actinomyces (47), E. coli (35)
Otitis Media/Interna
Pasteurella multocida (51)
Other bacteria
Joint Infections
Polyarthritis (hematogenous)
Monoarthritis/pauciarthritis (mainly hematogenous in dog, penetrating bite wounds in cats)
Viral: effusive feline infectious peritonitis (10), feline calicivirus (14), feline foamy virus (15)
Rickettsial: granulocytic Ehrlichia (26), Rickettsia rickettsii (27), Chlamydia (29)
Mycoplasmal: Mycoplasma (32), bacterial L-forms (32)
Bacterial: hemolytic: Streptococcus, Proteus, Pseudomonas, Erysipelothrix, Corynebacterium (33), Staphylococcus (34), E. coli (35), Salmonella, Yersinia (37), Brucella (38), anaerobes (39), Borrelia (43), Pasteurella (51, 87), Nocardia (47), Mycobacterium (48)
Fungal: Blastomyces (57), Histoplasma (58), Cryptococcus (59), Coccidioides (60), Aspergillus (62), Candida (64)
Protozoal: Leishmania (73)
Some cases of immune-mediated arthritis are associated with an infected focus elsewhere in the body
Myositis
Polymyositis
Viral: feline immunodeficiency virus (12)
Bacterial: Leptospira (42), Borrelia (43), Clostridium (39)
Fungal: Sporothrix (61)
Protozoal: Trypanosoma (72), Leishmania (73), Hepatozoon (74), Toxoplasma gondii (79), Neospora caninum (79), Sarcocystis (80)
Local myositis: numerous bacteria, toxigenic Streptococcus canis (33)
Osteomyelitis
Etiology
Pathogenesis
Hematogenous Spread
Posttraumatic Injuries
Biofilm
Clinical Findings
Metaphyseal Bacterial Osteomyelitis
Diagnosis
Therapy
Hematogenous Osteomyelitis
Organism (Chapter Reference)
Usual Condition
Systemic Antimicrobialsa
Mycoplasma (32)
A
Tetracycline,b quinolones
Streptococcus sp. (β-hemolytic) (33)
D, A, I
Ampicillin, penicillin,c clindamycin, first-generation cephalosporin
Erysipelothrix (33)
D, A
Penicillind,e
Staphylococcus coagulase negative (34)
O
First-generation cephalosporin, nafcillin
Staphylococcus pseudintermedius (34)
D, A, O
Amoxicillin-clavulanate, first-generation cephalosporin,d oxacilline
Actinomyces (47)
D, O
Penicillin
Proteus, Pseudomonas, E. coli (35)
I, O
Quinolones, aminoglycoside, second- or third-generation cephalosporins, ticarcillin-clavulanate, imipenem
Brucella canis (38)
D
Lipid soluble tetracyclineb and streptomycin,f quinolone
Anaerobes (39)
M, O, I
Amoxicillin-clavulanate, clindamycin, metronidazole, penicillin
Borrelia (43)
A
Ampicillin, tetracycline
Pasteurella multocida (51, 88)
I
Ampicillin, tetracycline
Blastomyces (57), Coccidioides (60)
O
Itraconazole, ketoconazole, amphotericin B
Cryptococcus (59)
A
Itraconazole, ketoconazole, fluconazole, amphotericin B
Aspergillus (62)
D
Itraconazole, amphotericin B
Toxoplasma (79)
M
Clindamycin, pyrimethamine and sulfonamides, azithromycin
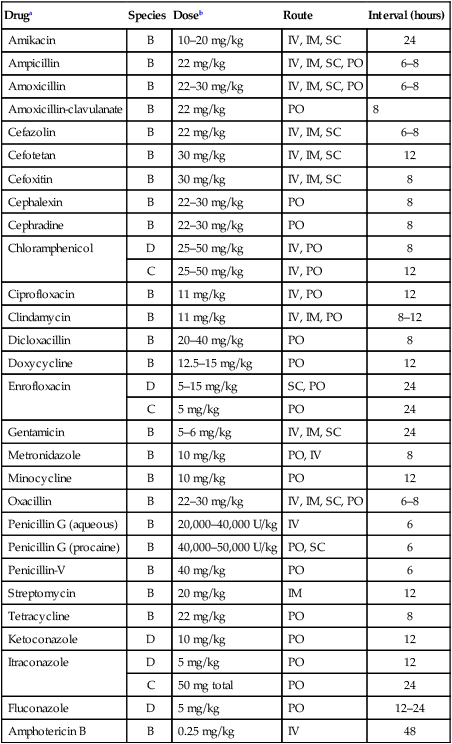
Druga
Species
Doseb
Route
Interval (hours)
Amikacin
B
10–20 mg/kg
IV, IM, SC
24
Ampicillin
B
22 mg/kg
IV, IM, SC, PO
6–8
Amoxicillin
B
22–30 mg/kg
IV, IM, SC, PO
6–8
Amoxicillin-clavulanate
B
22 mg/kg
PO
8
Cefazolin
B
22 mg/kg
IV, IM, SC
6–8
Cefotetan
B
30 mg/kg
IV, IM, SC
12
Cefoxitin
B
30 mg/kg
IV, IM, SC
8
Cephalexin
B
22–30 mg/kg
PO
8
Cephradine
B
22–30 mg/kg
PO
8
Chloramphenicol
D
25–50 mg/kg
IV, PO
8
C
25–50 mg/kg
IV, PO
12
Ciprofloxacin
B
11 mg/kg
IV, PO
12
Clindamycin
B
11 mg/kg
IV, IM, PO
8–12
Dicloxacillin
B
20–40 mg/kg
PO
8
Doxycycline
B
12.5–15 mg/kg
PO
12
Enrofloxacin
D
5–15 mg/kg
SC, PO
24
C
5 mg/kg
PO
24
Gentamicin
B
5–6 mg/kg
IV, IM, SC
24
Metronidazole
B
10 mg/kg
PO, IV
8
Minocycline
B
10 mg/kg
PO
12
Oxacillin
B
22–30 mg/kg
IV, IM, SC, PO
6–8
Penicillin G (aqueous)
B
20,000–40,000 U/kg
IV
6
Penicillin G (procaine)
B
40,000–50,000 U/kg
PO, SC
6
Penicillin-V
B
40 mg/kg
PO
6
Streptomycin
B
20 mg/kg
IM
12
Tetracycline
B
22 mg/kg
PO
8
Ketoconazole
D
10 mg/kg
PO
12
Itraconazole
D
5 mg/kg
PO
12
C
50 mg total
PO
24
Fluconazole
D
5 mg/kg
PO
12–24
Amphotericin B
B
0.25 mg/kg
IV
48
Acute Posttraumatic Osteomyelitis
Chronic Posttraumatic Osteomyelitis
Prevention
Diskospondylitis and Vertebral Osteomyelitis
Etiology
Dog
Cat
Pathogenesis
Clinical Findings
Dog
Diagnosis
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Musculoskeletal Infections