CHAPTER 8 LIZARDS
COMMON SPECIES KEPT IN CAPTIVITY
Lizards are members of the class Reptilia, subclass Lepidosauria, order Squamata (Box 8-1). They are further classified under the suborder Sauria, which distinguishes them from the snakes, worm lizards, and Tuataras (Sphenodon punctatus). There are more than 4000 known species of lizards.1 Lizards are one of the most diverse and successful reptile groups living today and exist in a variety of habitats, including arboreal (e.g., chameleons, geckos, iguanas), aquatic (e.g., Galapagos marine iguana [Amblyrhynchus cristatus], crocodile lizard [Shinisaurus crocodilurus]), fossorial (e.g., skinks, limbless lizards, blind lizards), and terrestrial (e.g., monitors, agamids, iguanids). Most lizard species have the ability to live and survive in multiple habitats but have a preference for which environment they spend most of their time.
BOX 8-1 Taxonomy of Lizards
Family Chamaeleonidae (Chameleons)
Subfamily Corytophaninae (Casquehead lizards)
Subfamily Crotaphytinae (Collared and Leopard lizards)
Subfamily Hoplocercinae (Wood lizards and Clubtails)
Subfamily Iguaninae (Iguanas and Spinytail iguanas)
Subfamily Oplurinae (Madagascar iguanids)
Subfamily Phrynosomatinae (Earless, spiny, tree, side-blotched, and horned lizards)
Subfamily Polychrotinae (Anoles)
Subfamily Tropidurinae (Neotropical ground lizards)
Family Pygopodidae (Legless lizards)
Family Dibamidae (Blind lizards)
Family Cordylidae (Spinytail lizards)
Family Gerrhosauridae (Plated lizards)
Family Gymnophthalmidae (Spectacled lizards)
Family Teiidae (Whiptails and tegus)
Family Lacertidae (Lacertids, wall lizards)
Family Anguidae (Glass lizards and alligator lizards)
Family Anniellidae (American legless lizards)
Family Xenosauridae (Knob-scaled lizards)
Infraorder Platynota (Varanoidea)
Family Helodermatidae (Gila monsters)
Family Lanthanotidae (Earless monitor lizards)
ANATOMY
Integumentary System
Like other reptiles, most lizards have thick skin with varying degrees of scaling. Lizards have ectodermal scales that can be smooth or keeled depending on the species.2,3 Those species with keeled scales have a rough or spiny appearance. Some lizards may incorporate osteoderms, especially around the head region as is the case with gila monsters and beaded lizards (Heloderma spp.). Unlike snakes, which have a continuous shed, lizards shed their skin in patches. Appropriate humidity for the particular species is essential for proper shedding or ecdysis. Some species, such as chameleons (Chamaeleo spp.), are known for their ability to blend into their environmentbecause their skin contains chromatophores that allow changes in color. The mechanism of these changes is not fully understood, but they appear to involve a complex group of reactions based on their environment. Chameleons also have the ability to change colors based on their moods and perhaps even as a way to communicate with others chameleons and fend off potential predators. Another way in which lizards have evolved and adapted to their environments is via ornamentation in the form of coloration as well as skin extensions expressed as dewlaps, spines, crests, and skin folds.2 These features may help veterinarians differentiate males from females, whereas others are designed for protection against predators. Lizard skin is largely aglandular, although some species have femoral pores/glands on the ventral aspect of the thigh. In these species, the males have pores of considerable size whereas they are barely visible in the females (Figures 8-1 and 8-2). Others have both femoral and precloacal pores. This is one form of sexual dimorphism in lizards. The subcutaneous space of lizards varies with the species and should be a consideration at the time of fluid administration. Finally, some species have well-developed claws that can inflict damage on handlers. Therefore, regular trimming is recommended for those lizards in captivity.
Gastrointestinal System
TEETH
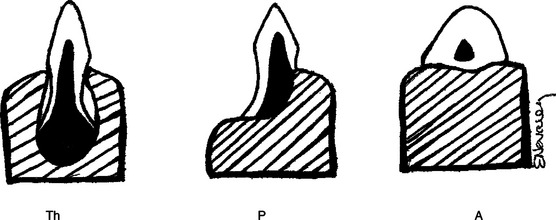
Lizard teeth are classified as pleurodont or acrodont. Pleurodont teeth have longer roots with weak attachments to the mandible and no socket (Figure 8-3). They rest on the lingual side of the mandible; the buccal side has a prominent ridge of bone. Pleurodont teeth can be replaced throughout the life of the lizard.2 Acrodont teeth have shorter roots with a firmer attachment, lack sockets (see Figure 8-3), and are fused with the bone itself. Acrodont teeth are not replaced in adulthood.2 These considerations should be taken into account when performing oral examinations so as to avoid damage to the teeth, especially those with acrodont dentition. The teeth of lizards have varied functions depending on the species. In some lizards, they aid in the grinding of coarse food material before passing to the stomach. Other lizards rely on their teeth to tear or break larger pieces of food into smaller pieces that are then swallowed whole. Teeth also play a role in defense from predators and during mating. Some males latch on to the females during copulation by biting them on the neck or head. Nonetheless, the teeth of most lizards probably do not play as important a role as they do in other groups of animals. The size of lizards’ teeth is small compared with the size of their heads and bodies.
Cardiovascular System
Lizards have a three-chambered heart comprised of two atria and a single ventricle. An anatomic-physiologic body function of lizards and other reptiles, which has raised some controversy in the veterinary literature, is the renal portal system (RPS). Under certain physiologic conditions, the RPS allows blood from the caudal aspect of the body to circulate through the kidneys and/or liver before reaching the systemic circulation. This renal portal blood flow has been perceived as a problem for drug administration. Some drugs may be nephrotoxic and lead to adverse effects if carried directly into the kidneys. The metabolism of drugs also may be affected if they pass through the kidneys before generalized tissue distribution, resulting in the drugs being excreted before reaching the target tissues. Studies have investigated the RPS in red-eared sliders (Trachemys scripta elegans)4 and green iguanas.5 Both investigations concluded that blood flowing from the hind limbs is directed primarily at the liver, bypassing the kidneys. In red-eared sliders, blood flowing from the tail region was shown to flow into the kidneys or bypass the kidneys and go straight into the liver.4 In green iguanas, blood flowing from the tail was shown to flow into the kidneys.5 Benson and Forrest stated that “the renal portal vasculature joins the primary renal circulation at the level of the efferent arterioles.”5 This would imply that drugs excreted by glomerular filtration would be spared by the RPS, whereas those excreted by tubular secretion would be adversely affected.5 This idea is further supported by findings of a study in red-eared sliders that evaluated the effects of the RPS on the pharmacokinetics of gentamicin and carbenicillin.6 In mammals, gentamicin is excreted via glomerular filtration and carbenicillin through tubular secretion. After comparison of cranial and caudal injections of these drugs, it was concluded that gentamicin pharmacokinetics was not affected by site of administration.6 However, carbenicillin was observed to have a perceived reduction in bioavailability after injection in the caudal limbs as opposed to the cranial limbs.6 This reduction in bioavailability was determined not to have clinical relevance. A first-time passage through the renal tubules for a drug excreted at the tubules would explain the perceived decrease in bioavailability. Despite these findings, there is not enough evidence to support a clinically relevant decrease in the effectiveness of drugs administered in the caudal musculature of reptiles. Conversely, these studies are limited and do not take into consideration multiple variables that may affect the RPS. A valve system was proposed as a part of the control of the RPS but was not proven in all species.4,5 Factors affecting the metabolism of the reptile, such as temperature, nutrition, time of the year, and health status, may influence the physiologic mechanisms of the RPS and regulate when it will or will not direct blood flow into the kidneys and/or liver. Further studies are needed to evaluate the RPS under various conditions in lizards and other reptile species.
Renal System
Lizards, like most reptiles, are uricotelic. The end product of protein catabolism is the production of uric acid by the liver, which is then transferred over to a metanephric kidney. The kidney goes through three main stages of development: pronephric, mesonephric, and metanephric. In higher vertebrates, the mesonephric kidney dominates into adulthood and is comprised of an elaborate arrangement of nephrons with a well-developed renal pelvis and a loop of Henle that allows efficient urine concentration. The reptilian kidney, on the other hand, is dominated by the metanephric arrangement in which there are far fewer nephrons and a poorly developed renal pelvis. The reptilian kidney lacks a loop of Henle and therefore is not able to concentrate urine as a mesonephric kidney would. Uric acid is filtered by the glomeruli and secreted by the proximal tubules.7 Because of this, lizards are not able to concentrate urine beyond the osmolarity of their plasma.7 The urine follows a path through the mesonephric duct into the urodeum. Most lizard species have a urinary bladder, which receives urine from the urodeum. Together with the liquid urine, there is excretion of urates, which are the insoluble salts that aid in the conservation of water.
The posterior segment of some lizard kidneys express sexual dimorphism during the reproductive period, increasing in size to produce seminal fluid in males.2 The uricotelic nature of reptiles allows them to conserve fluids in a way different from other animal groups. Reptiles, in general, excrete urates and retain as much fluid as possible. This adaptation is essential for those animals living in desert environments. However, this adaptation also contributes to the difficulties of managing renal disease in reptiles.
Reproductive System
Sex determination may not be easily accomplished in some reptile species. Others have sexual dimorphism that allows differentiation of males and females based on external characteristics. In the green iguanas, males tend to have large heads with big dewlaps, operculum scales, and dorsal spines, all considered to be male-associated characteristics. Females have smaller heads and smaller dewlaps. Other lizard species may also have increased ornamentation in the males in the form of colors, crests, spines, casques, and other exaggerated external features as compared with the females (Figures 8-4 and 8-5). This distinction in masculine versus feminine characteristics is not evident in all lizard species. Instead, veterinarians must rely on more precise ways to differentiate the sexes. Large femoral pores can easily be observed in the adult male of many species (see Figure 8-2). Females have smaller femoral pores as compared to the males (see Figure 8-1). Another distinction comes from the presence of the male copulatory organs, the hemipenes, which are located on the ventral aspect of the base of the tail caudal to the location of the vent. A hemipenal bulge can be observed on adult males of some species. The hemipenes are a purely copulatory organ with no association with the urinary system. Therefore, the copulatory organs can be amputated in cases of trauma or unresolved paraphimosis. This occurs with lizards and other reptiles.

Figure 8-4 Compared with male marine iguanas, female marine iguanas have smaller heads and darker coloration.
Nervous System
To treat a reptile with neurologic signs, it is important to be knowledgeable of the animal’s normal behavior, both in the wild and in its captive environment. This will help determine if the clinical signs, as described by the owner, are abnormal. Although muscle tremors may be diagnosed as a neurologic problem, a veterinarian must consider primary underlying causes such as a dietary calcium deficiency. Head tilt, abnormal posture and ambulation, slow righting reflex, and lack of awareness of their environment are some of the more obvious neurologic signs that may be observed in affected reptiles. Others signs, such as decreased proprioception, pupillary light reflexes, and strength, may be more difficult to assess. A thorough history is needed when trying to assess exposure to toxins and trauma. Reptile patients with metabolic disorders may also present with neurologic signs (e.g., secondary nutritional hyperparathyroidism). Diagnosis of primary neurologic disease is rare in reptile species maintained as pets and often is determined after ruling out other disease processes.
Respiratory System
Nostrils are clearly visible in most lizards. Nasal salt glands also are present in some lizard species (e.g., green iguanas). A prime example of nasal salt glands can be found in the Galapagos marine iguanas, which have extensions from their nostrils to aid in the expulsion of salt away from the body (Figure 8-6). Lizards have a single tracheal opening (glottis) located at the base of the tongue. With the mouth closed, the glottis is in close apposition to the internal opening of the nares, similar to the arrangement of the choana in birds. The lungs of lizards have evolved over time whereby the lung structure can be air sac–like and lined with a minimal number of falveoli, or a more elaborate, reticulated tissue with a higher number of falveoli. In either case the lung structure is less elaborate than in mammals. The lungs usually comprise a significant volume of the coelomic cavity and can extend caudally into the lumbar region of some lizard species during inspiration. Lizards also use their lungs as air reserves to inflate their bodies and remain wedged between rocks to defend against a predator attack; they may display this behavior during a physical examination. Because they lack a diaphragm, lizards rely on the mechanical action of the ribs during inspiration and expiration for respiration to occur. Care must be taken to avoid a tight grip around the body of lizards, as this may interfere with respiration. Auscultation of normal lungs should reveal minimal respiratory noise. Increased respiratory noises may be due to normal vocalization (e.g., hissing) or a true anomaly (e.g., pneumonia). Diagnostics such as radiographs and blood work may be used to investigate a suspected anomaly.
Auditory System
Most lizards have external ear openings, and in many species an external tympanic membrane may be observed (Figure 8-7). This membrane is usually translucent and may be covered by skin. The posterior side of the membrane should appear dry with no indication of fluid accumulation. Behind the tympanic membrane lies the middle ear cavity and structures of the inner ear with their vestibular function. These structures are as varied as the species of lizards in which they are found. Ultimately, the hearing of lizards may be limited to detecting vibrations from their environment, with little acuity for noise. There is little vocalization among the many lizard species, but some may communicate in frequencies outside of the range of human hearing. From a clinical standpoint few diseases of the ear are recognized in lizards. Aural abscesses are a common clinical finding in certain tortoise species but are rarely noted in lizards.
Ocular System
The anatomy of the lizard eye is quite varied and interesting. Some species have eyelids, whereas others rely mostly on spectacles for protection of the cornea. Lizards with eyelids may also have a nictitating membrane. Species without eyelids (e.g., gecko) may use their tongues to keep the spectacle/cornea clean and moist. Some species also possess scleral ossicles, similar to those present in many bird species, which maintain the shape of the globe. The cornea lacks a Descemet’s membrane, and the iris is controlled primarily by striated muscle (similar to avian species), precluding the diagnostic return of pupillary light reflexes.2 The shape of the pupil varies with the species. Diurnal species usually have larger round pupils, whereas nocturnal species usually have vertical pupils that are able to constrict to a very small size. Most species have avascular retinas and a conus papillaris.2 A pineal eye, not a visual structure is present in some species (Figure 8-8) and is responsible for detecting variations in light cycles and may aid with thermoregulation through determining optimum basking periods. Some lizards have very special adaptations to their eye structure. For example, chameleons have the ability to moveeach eye independently, whereas the horned lizard (Phrynosoma spp.), can squirt blood from its periocular tissue to fend off predators. Overall veterinarians must be aware that ocular diseases occur with some frequency in reptiles.
HUSBANDRY
Environmental Considerations
ENCLOSURE TYPE AND SIZE
The rule of thumb for enclosure size is the bigger the better, with an underlying consideration to plan for an appropriate enclosure size for the adult lizard. Often an appropriate enclosure size is provided for juvenile lizards, but they quickly outgrow this space. The main orientation of the enclosure depends on the species. Arboreal lizards benefit from enclosures with a vertical orientation (Figure 8-9), whereas terrestrial and fossorial lizards require enclosures with a horizontal orientation (Figure 8-10). In addition to the size of the enclosure, construction materials must be appropriate. Synthetic nonporous materials are preferred, as they are easier to clean and lighter in weight. However, cages made of these materials can be expensive and need to be purchased from a manufacturing company. Wood is still a popular material for the construction of reptile enclosures. Wood is readily available and inexpensive, and most people can build a lizard’s enclosure. The main disadvantages of wood are its weight and its ability to retain water and organic matter. Pressure treated wood that contains chemical compounds to prevent decay must be avoided because of potential toxin exposure. Wood used in reptile enclosures must be properly sealed against water damage by using stains and polyurethane. It is also advisable to seal the seams and corners with a silicon caulking material for ease of cleaning. The wood stains, polyurethane, and caulking must be allowed to cure for several days to avoid any toxicity. It is important to inspect the enclosure on a regular basis to ensure that the wood is completely sealed and intact. A more durable, safe but expensive option is the use of synthetic decking material that is now available. Plexiglas® is a lightweight material that may be used in combination with wood to create enclosures for lizards. Glass tanks can make adequate enclosures for juvenile lizards and those species that mature to a smaller size (e.g., anoles). Glass tanks must have screen tops to provide proper ventilation. One material that should be avoided when building an enclosure is uncoated metal wire mesh. This material will lead to skin abrasions when the animals rub against or climb on it. Also, galvanized wire has the potential of causing zinc toxicity in lizards if they chew on the wire and accumulate toxic levels of the metal over time. A plastic coated wire mesh is available and appropriate for use in enclosures. The size of the wire mesh should be such that it does not cause injuries from escape attempts and does not allow other animals to enter the enclosure (e.g., domestic dogs or cats, wild raccoons). As a general rule, the size of the mesh opening should be smaller than the size of the head of the animal. One major advantage of using mesh is than the enclosure is well ventilated. In addition, wire mesh is lightweight and relatively inexpensive.

Figure 8-10 Horizontal enclosure ideal for fossorial and terrestrial lizards, such as skinks and bearded dragons.
TEMPERATURE AND HUMIDITY
Reptiles rely primarily on the environmental temperature to regulate their internal body temperature. In a natural environment there are normal variations in body temperature during a 24-hour period. Many reptile species take advantage of the early morning and mid-day sun to bask and warm their bodies. As the day progresses, the reptile’s internal body temperature begins to decrease as the environmental temperature decreases. I have observed greater than 20° F variations in the cloacal temperature of green iguanas over a 12-hour period. This apparently wide temperature gradient is part of the normal biology of many lizard species. Reptiles and lizards strive to achieve an optimal body temperature during the day, which is essential for the physiologic processes of digestion, immune function, acid-base balance and reproduction.8 Ultimately, the animal must maintain an adequate metabolic rate to sustain homeostasis. The temperature at which a lizard has an adequate metabolic rate sustaining homeostasis has been referred to as the preferred optimal temperature zone or the thermal neutral zone.8 Regardless of which term is used, reptiles have an optimal temperature, a single temperature, at which physiologic function is at its peak. In an ideal situation this temperature would be achieved for a certain period of time every day, but in reality most reptiles thrive within a range of acceptable temperatures that allow them to maintain appropriate physiologic function. Ultimately, achieving an acceptable temperature range is the goal of temperature regulation for captive lizards, allowing for diurnal variations in temperature and a reduction in the nocturnal temperature. Although actual requirements vary among lizard species, some generalizations can be made about the most common species maintained in captivity. A recommended diurnal temperature range for tropical and desert lizard species is 85° F to 95° F, with a basking light provided to allow for a concentrated area of heat (reaching a maximum of 100° F to 105° F) in the area directly underneath it. To avoid thermal burns on the animal, it is essential to monitor the temperature under the basking light. There should always be a barrier between the light and the animal, with enough room to allow basking as desired. The nocturnal temperature range for most species should be between 72° F and 78° F. Ceramic heat emitters (Figure 8-11) and basking lights (Figure 8-12) are good sources of radiant heat. Various types of heating pads are available for use under the reptile enclosures, with most having an adhesive that attaches them to the underside of the enclosure. The most important consideration regarding the heating pads is to ensure that they are calibrated to achieve a maximum safe temperature or that a rheostat is used to control the heat output. A layer of substrate is recommended between the heated area and the animal for added protection against thermal burns. Most veterinarians suggest leaving the heating pad on at all times and utilizing the basking lights or ceramic heaters to increase the diurnal temperature. The room temperature also can be regulated to provide an adequate environment. If the room is maintained at a warmer temperature, it will be easier to achieve the desired temperature within the enclosure. Various types of thermometers are available to monitor temperature inside reptile enclosures. Some thermometers record temperatures at different intervals or providemaximum and minimum temperatures, giving the owner a daily temperature gradient within the enclosure.

Figure 8-12 Basking lights provide both radiant heat and visible light, but most have no ultraviolet B radiation output.
LIGHTING
Lighting requirements of reptiles vary with the nutritional requirements of the species in question. It is generally thought that herbivorous, insectivorous, and most omnivorous reptiles have an absolute requirement for ultraviolet (UV) radiation, specifically in the UVB spectrum. Light in the UVB spectrum is essential for the synthesis of vitamin D3 and consequently the absorption and metabolism of calcium. Nonetheless, there are no specific requirements for these species to indicate how much exposure they really need. Carnivorous species are thought to have the ability to obtain enough vitamin D3 from their prey; however, studies have shown that carnivorous species may still benefit from exposure to UVB.9 The best source of UVB is direct, unfiltered sunlight. Owners must remember that glass and other materials filter beneficial UVB rays. Lizards in captivity maintained indoors should be exposed to natural sunlight 2 to 3 times a week, especially in the summer, in addition to artificial UVB. During the winter an owner may need to rely only on artificial sources of UVB. Many sources of artificial UVB lights are available in the market. Although many of these bulbs are referred to as full spectrum, owners must make sure that the one purchased has an appropriate output in the UVB spectrum. There are two main types of bulbs that provide UVB: fluorescent bulbs and mercury vapor bulbs. Fluorescent bulbs come in a variety of UVA and UVB percentage outputs. The main output of consideration should be the UVB, which should be at least 5%. Fluorescent bulbs are available as long tubes or strip lights and as a coiled bulb version that can be used with regular incandescent light fixtures (Figure 8-13). The type for use with incandescent light fixtures shows promise of providing a more effective UVB output.10 The average price of a fluorescent bulbwith UVB spectrum is approximately US$25 to $35. One disadvantage of fluorescent bulbs is their effective working distance. Most bulbs will provide adequate UVB within 12 inches from the bulb, but the UVB output decreases exponentially beyond 12 inches from the bulb. This dramatic reduction in UVB radiation is an important consideration when designing the enclosure. In enclosures taller than 12 inches, a climbing surface must be provided to allow the lizard to get within 12 inches of the bulb. Placing a basking light nearby will ensure that the animal effectively uses the area under the light. These bulbs usually have an effective life span of 6 months, at which point they must be replaced by a new bulb, whether or not they still provide light.
SUBSTRATE
The choice of substrates for reptile enclosures is a main subject of discussion among veterinarians and owners. The primary substrate consideration should be a nontoxic, easily digestible product that is absorbable and easy to clean. Unfortunately, most commercially available products do not meet these requirements. In my opinion, the best substrates are newspaper, other nontoxic paper, or artificial turf (also known as indoor/outdoor carpeting). Newspaper is inexpensive, nontoxic, easy to clean, and, if ingested, easily digestible. Unfortunately many owners do not find newspaper to be very esthetic. Newspaper as a substrate also does not meet the goal of creating a natural vivarium. Artificial turf is readily available in green and brown colors, thereby being more aesthetically pleasing while meeting the necessary criteria for a substrate. The artificial turf must be of good quality and not the type that will lose strands of material easily, as these pieces may be ingested and result in intestinal blockage. Other commercially available products that may be recommended include recycled paper products, ornamental bark chips (not cedar or pine), aspen bedding, and cypress mulch. All substrate products have the potential of being ingested by the animal and causing an obstructions. Some substrates hold more moisture and are more difficult to clean. One consideration in choosing a substrate is that the individual substrate particle should be too large for the lizard to swallow. Accidental ingestion usually occurs while feeding the lizards; therefore, an appropriate feeding bowl or container should be provided that is deep enough to prevent spillage of the food within it. Alternatively, owners can try to feed the lizard in a separate container with no substrate in it; although well-acclimated lizards may tolerate this, for most lizards it would be a stressful situation. There are some products that should not be used as substrate in reptile enclosures, including cedar chips, cat litter, corn cob, and other products sold as a digestible reptile substrate, such as calcium carbonate sand. The so-called digestible sands sold in many pet stores are not as digestible as advertised and may cause severe impactions when ingested. They are also advertised as a source of calcium for reptiles, but this is misleading. (Proper sources of calcium are discussed in Nutrition.) Many veterinarians have treated cases of gastrointestinal impaction in lizards that ingested sand substrate. For experienced breeders or hobbyists that have the time and knowledge to monitor their collection very closely and desire a sand substrate, one alternative is the use of play sand that has been sifted very carefully to remove the larger particles. Sand substrate should be reserved for adult specimens only, because young lizards are more curious and may be more prone to accidental ingestion. In addition, newborn and juvenile animals have a smaller diameter to their intestinal tract, and even a small amount of sand can cause gastrointestinal obstruction. Novice hobbyists should avoid the use of all sand products. Although many people have successfully used sand and other particulate substrates over the years, these materials continue to be a cause of intestinal impaction for many lizards maintained as pets.
NUTRITION
Herbivores
Herbivorous reptiles have a primary dietary requirement of plant material, including grasses, leaves, and fruits. Most herbivorous reptiles have a colon that aids in the fermentation of feedstuffs and require a diet with moderate to high fiber content and moderate to low fat and protein content. Not all herbivorous lizards have the same nutritional requirements, and it is incumbent on the owner to become knowledgeable about the particular requirements of the species being maintained in captivity. Even with green iguanas there are many variations with respect to dietary recommendations. Therefore, a general recommendation for feeding herbivorous lizards will be presented. Specifics for each species of lizards can be located in other published sources. A common mistake is feeding the captive herbivorous lizard too many carbohydrates in the form of fruits and/or diets too high in protein (e.g., dog or cat food). The key management point is to introduce a balanced diet that not only provides fiber, protein, and fat but also other nutritional elements, such as vitamins and minerals. The source of the protein is also an important consideration, because for herbivorous reptiles, the protein source should be of primary plant origin. Allen and Oftedal’s recommendations for the dietary requirements of green iguanas consist of 22% to 26% protein level on a dry matter basis for juvenile iguanas and 15% to 17% protein on a dry matter basis for adult, nonreproductive iguanas.11 This is accompanied by recommendations of 6% to 10% crude fiber or 10% to 18% acid detergent fiber.11 At first this may seem contraintuitive to what herbivores should require, but these recommendations are based on a primary diet of plant matter. The protein content of plants is smaller when compared to animal sources and it is also not as digestible. This explains why, on a dry matter basis, the protein requirement is larger than fiber requirements and why the protein requirement would be smaller if the source of the protein was more easily digestible. Baer et al. found that diets with 12% crude fiber or 20% acid detergent fiber led to decreased weight gain and energy retention in juvenile green iguanas.12 A proposed explanation for this observation is that the iguana’s digestive system is not as specialized as that of ruminants and may not be able to efficiently process dietary fiber. An additional observation is that little grinding of the plant material occurs before ingestion; therefore, the material reaches the intestinal tract with a large particulate size that is harder to digest.11 Perhaps offering finely chopped plant material would help the digestibility of the fiber in the intestinal tract of herbivorous lizards. The nutrition of green iguanas has been studied more than that of most lizard species. The same principles may or may not apply to other herbivorous lizards, depending on their natural habitat, available feedstuffs, and efficiency of their digestive tract. A critical aspect of the nutrition of herbivorous reptiles is the ability to maintain an overall balance of calcium and phosphorus at a ratio of 1.5 : 1 to 2 : 1, which is essential for optimum calcium availability and metabolism.
Even with different views of what and how to feed herbivorous lizards, the primary recommendation is to offer as varied a diet as possible. Some commercial diets are available for different lizard species, but many are limited in their nutritional composition. Also the nutritional analysis of these commercial diets is not regulated and is often inaccurate.11 Commercial diets should not be used as the sole source of feed for any lizard species. Some companies have the input of properly trained nutritionists and herpetologists to assist in the manufacture of their commercial feed products, whereas others do not. Some diets are readily available to the public, whereas others may only be purchased by zoologic institutions or sold in bulk quantities. The alternative to commercial diets is feeding what is known as the “salad-based diet.” The salad-based diet consists of different greens and vegetables from the local grocery store or produce stand. The variety of vegetables offered varies according to season and geographic location. A list of some greens and vegetables that are recommended for feeding herbivorous reptiles is provided in Box 8-2. A controversial item in this table is the feeding of lettuce (red or romaine). It is argued that red and romaine lettuce have little nutritional value and therefore should not be fed to reptiles. Although the concern about lettuce has its merits, these items should be offered as part of a mixed, varied diet and not as a sole source of plant matter. Red and romaine lettuces are both readily available and represent a dietary source of fiber that can be used as part of a balanced diet. The more diversity that can be offered, the more balanced a diet is likely to be. In addition toitems that may be purchased at the grocery store, the local garden store will sell some plants with leaves and flowers or fruits that may be used to feed reptiles. Both the flowers and leaves of the hibiscus plant can be offered to herbivores and are part of the natural diet of some tropical lizards. Dandelions are a favorite of most reptiles and can be offered as part of a varied diet. All plants offered to reptiles must be free of pesticides and fertilizers to avoid potential toxicities. A controversial dietary recommendation is what source of protein should be made available to herbivorous reptiles? If iguanas are considered to be true herbivores, then the protein should come from a plant source. Alfalfa has been advocated as an ideal source of protein for reptiles. Alfalfa can be found as the fresh plant (not the sprouts), pellets formulated for mammals, and even tablets for humans. Although alfalfa is likely the best source of plant protein, it should not constitute the majority of any reptile’s diet. Like other food items, alfalfa should be used as a component of the dietary plan.
Although there is a general consensus on what not to offer nutritionally, few are in agreement on what to feed herbivorous lizards. High-protein dog and cat foods should not be offered to herbivorous lizards. Some expert hobbyists and herpetologists have included dog or cat foods as part of the dietary plan for certain lizards, which is done under a closely observed nutritional plan that takes into consideration the amount, frequency, and nutritional value of all items fed. For the average pet lizard owner, this nutritional oversight can be an overwhelming task and many times leads to excessive feeding of a high-protein diet, which, in turn, may lead to renal disease. Some leafy greens and nonleafy vegetables are rich in oxalic acid. If ingested in excessive amounts, oxalic acid can bind calcium and reduce the absorption of this important mineral. Food items with high oxalic acid (OA) content should be limited (e.g., parsley (1.7 g OA/100 g), spinach (.97 g OA/100 g), and chives (1.48 g OA/100 g)).13 Alfalfa also is known to have increased levels of oxalic acid and therefore should be fed in moderation. Other greens and vegetables may contain high amounts of glucosinolates. These are goitrogenic glycosides present in some plants that have been associated with toxicity.14 Plants with high levels of glucosinolates are being studied for potential benefits in treating cancer (e.g., cabbage, Brussels sprouts, broccoli, cauliflower, kale, kohlrabi, Swiss chard, radish, pak choy, and watercress).14 Plants with high glucosinolates should also be used sporadically and not as a main ingredient in the diet.
Feeding herbivorous reptiles is complex and full of contradictions, depending on the reference sources. No plant material by itself is nutritionally balanced, and some may cause additional problems if fed frequently and/or in large amounts. The best and most simple approach to feeding herbivorous reptiles is to provide a combination of fresh dark leafy greens, vegetables, plants (e.g., leaves, flowers, and fruits), alfalfa, commercial diet, and vitamin/mineral supplementation. Plant material should constitute 50% to 80% of the diet. The rest of the diet should be a commercial formula deemed appropriate for the species being fed. Alfalfa can also be added to the diet but should not be used in conjunction with food items containing high levels of oxalic acid. Vitamin and mineral supplements designed for reptiles can be sprinkled over the food with the amount limited to that of the allowed dose of vitamin A in reptiles (56 international units/kg body weight).11 Vitamin and mineral supplements should be used with caution when feeding a commercial diet to prevent nutritional toxicity or imbalance. All greens and vegetables should be thoroughly washed before being offered to remove organic debris and prevent contamination with bacteria and other organisms.
PREVENTIVE MEDICINE
Disinfection and Sanitation
Hygiene is an essential part of maintaining healthy animals, and with lizards, feces, urine, and food are the main sources of organic waste. The type of cage and substrate will influence the ease of cleaning the waste material. Most lizards defecate/urinate a few times per week, depending on the diet and frequency of feeding. Organic waste should be removed from the enclosure immediately. Any surface that comes into contact with the organic waste must also be cleaned and disinfected. A complete cleaning and disinfection of the enclosure, including removal of substrate and accessories, should be performed every 3 to 6 months. A mild soap solution or an organic nontoxic cleaner is recommended for cleaning and disinfecting the interior surfaces, accessories, and cage furniture. Chemical cleaners and disinfectants may prove toxic to the animal and therefore should not be used. There are some products sold for use in reptile enclosures, but the ingredients of these products must be examined carefully before their use. The most common disinfectant used is a 1 : 10 solution of bleach and water, applied after removal of all organic material. The bleach solution should be rinsed off and the enclosure ventilated thoroughly before placing the lizard back in the enclosure. Other acceptable disinfectants include quaternary ammonium compounds, chlorhexidine, and iodophores.15 Disinfectants must be used in a dilute solution and rinsed thoroughly after use in a reptile habitat. Finally, it is important to take appropriate measures to prevent cross-contamination of household items and surfaces bacterial contaminants (e.g., Salmonella spp.). Individuals must thoroughly wash their hands after coming in contact with a reptile and their environment. Avoid hand washing in the kitchen sink; use a bathroom sink instead, and if possible, wash the cage and accessories outside of the house. If no outdoor facility is available, thoroughly disinfect sinks and/or bathtubs after the accessories and cage are cleaned. An antibacterial disinfectant should be used on household surfaces that come in contact with reptiles and the contents of their enclosures. Children, the elderly, and immune-compromised individuals should consider additional measures if handling reptiles and their enclosures. These recommendations are aimed at preventing the transfer of zoonotic diseases from reptiles to humans.
RESTRAINT
Manual Restraint
Most lizard species can be restrained manually by a single individual. Larger species such as monitors and iguanas may require multiple individuals. Because a lizard may bite, its head should be restrained. Placing an index finger and thumb around the base of the mandible will secure the head. The handler’s free hand should be used to restrain the rear legs and the tail (Figure 8-14). Lizards should not be grabbed by their tail, as some species have the ability to lose the distal part of the tail as a defense mechanism; this is known as tail autotomy. Extra care must be taken when restraining species with autotomy(e.g., green iguanas). Some lizards will be accustomed to handling and may be placed with their ventrum resting on your forearm, but the handler must remain vigilant to avoid being bitten. A technique that may calm some species of lizards is to cover both eyes with cotton balls or gauze and wrap their heads with bandaging material. This will create vagal stimulation and lead to a calming effect that may be beneficial when obtaining radiographs or performing a physical examination.
PERFORMING A PHYSICAL EXAMination
The physical exam provides the initial health assessment of any animal, including the lizard. Veterinarians must be familiar with the normal anatomy of the lizard species being examined to differentiate between normal and abnormal findings. Experience and other resources will help veterinarians become familiar with individual differences between species. Once the animal is properly restrained, the physical examination should begin. The eyes are examined for any evidence of dryness, blepharospasm (in those species with eyelids), and asymmetry. Looking at the dorsal aspect of the head is useful in determining symmetry of the eyes. Sunken eyes are indicative of dehydration. The function of the third eyelid, if present, should be assessed. Some species will have external ear openings or tympanic membranes. Oral examination can be performed with the help of a speculum (e.g., tongue depressors, rubber spatulas, wood applicators). One must exercise caution when opening the mouth to avoid breaking the teeth. The appearance of the oral cavity is quite varied depending on the species. Look for evidence of asymmetry, which may be related to root tooth abscesses, ocular disease, trauma, or all of these things. It is common in most species to have accumulation of saliva in the caudal aspect of the pharyngeal cavity. This saliva can be slightly viscous at times and should not be confused with a sign of dehydration, as in mammalian species. The mucous membranes normally appear as pale pink, pink, or even pigmented depending on the species. Blood vessels in the oral cavity can be used to help assess hydration and perform a capillary refill–like test similar to that used with mammals. Although it may not be as accurate as in mammals, a slow refill of the vessel (greater than 3-5 seconds) should prompt further assessment of the hydration status. Cotton tip applicators may be used to press against the vessels and determine a capillary refill time.
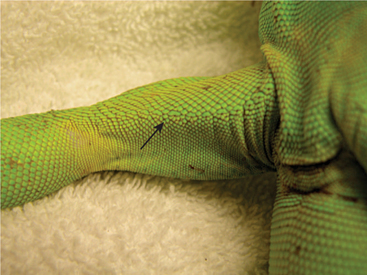
The examiner should assess the condition of the skin. Scales should be flat against the skin with a uniform overall appearance. Areas of abnormal pigmentation with raised skin or scalesand a sharp line of demarcation may be indicative of fungal or bacterial dermatitis (Figure 8-15). These areas should not be confused with normal shedding, which, in lizards, occurs in a nonuniform pattern over time. The examiner also must look for evidence of external parasites such as ticks and mites (Figure 8-16). Ectoparasites are commonly found between the scales/skin under the mandible, axillary and inguinal regions, and at the base of the tail.

Figure 8-15 Dermatitis on the head of a bearded dragon. This animal was housed outdoors in a subtropical environment.
DIAGNOSTIC TESTING
Clinical Pathology
Clinical pathology of lizards is used on an everyday basis to help diagnose diseases and monitor response to therapy. CBCs and chemistry panels (plasma or serum) are two common tests that are highly recommended to determine a lizard’s baseline health status. In addition, clinical pathology involves the examination of cytologic specimens and urine samples as well as other tests like protein electrophoresis. Veterinarians must explain to the clients the importance of performing these tests and how they will help monitor the patient’s health. The veterinarian should also be aware of the factors that may influence the results of these tests (e.g., species, age, sex, reproductive status, and season of the year).
Venipuncture
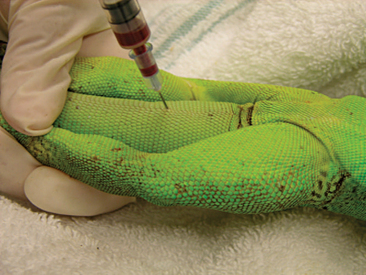
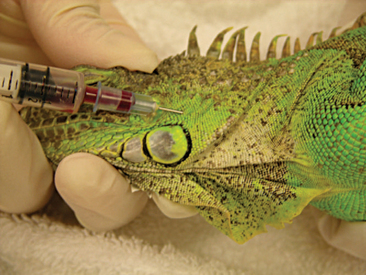
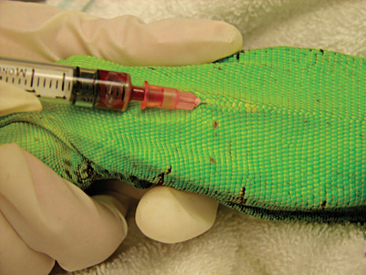
Venipuncture can be accomplished with a 1- to 3-ml syringe and a 22- to 26-gauge needle depending on the size of the animal and the collection site. There are three main sites used to collect blood from lizards. Site selection depends on personal experience, size of the animal, species, and health of the animal. The ventral coccygeal vein can be accessed from either the ventral or lateral aspect of the tail (Figure 8-17). This vessel lies along the ventral midline of the coccygeal vertebrae. Hypothermia and hypovolemia may increase the difficulty of a blood draw from the ventral coccygeal vein because of peripheral vasoconstriction. The jugular vein is located on the lateral aspect of the neck from a point near the caudal mandible to the point of the shoulder (Figures 8-18 and 8-19). In lizard species with an external ear opening, the opening or tympanic membrane can be used as the cranial landmark. Jugular venipuncture in lizards is usually a blind procedure with the needle being inserted at a location based on the anatomic landmarks described earlier. The third site for blood collection in lizards is the ventral abdominal/coelomic vein, which is located on the ventral midline. This vessel bifurcates at the umbilicus; therefore, venipuncture must be attempted cranial to the umbilicus (Figure 8-20). Cardiac puncture is not recommended in lizards.
Blood Handling
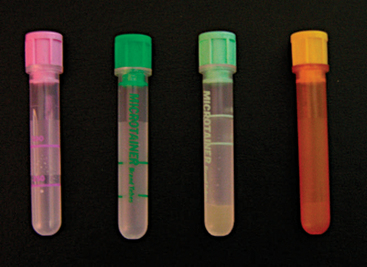
The sample must be handled carefully to avoid hemolysis, which will affect the results of the CBC and chemistry panel. Hemolysis can damage the integrity of the cells and lead to difficult interpretation of the differential count and artificial decreases in the total white blood cell (WBC) count. Hemolysis was shown to affect plasma levels of phosphorus, potassium, total protein, and aspartate aminotransferase in 10 green iguanas.16 The blood cells of many exotic species are fragile and have a tendency to clot quickly. To obtain a better sample, one should remove the needle from the syringe before transferring the blood into the tube. The blood must be transferred immediately after collection and mixed well with the anticoagulant to avoid clotting. Because the blood volume that can be collected safely from lizards tends to be very small compared to that from mammalian species, the use of Microtainer® blood tubes (BD Vacutainer Systems, Becton Dickinson and Company, Franklin Lakes, NJ) (Figure 8-21) is recommended. These tubes require a minimum of 0.25 ml and a maximum of 0.5 ml of blood for an appropriate ratio of blood to anticoagulant. For the majority of reptiles (except chelonians), a sodium ethylenediaminetetraacetic acid (EDTA) tube (purple top) will be appropriate. EDTA has been shown to provide better results than lithium heparin in samples obtained from 32 green iguanas.17 Lithium heparin (green top) can also be used if EDTA tubes are not available, but the cells will be more susceptible to degradation. Lithium heparin tubes come in two varieties. One has a gel that will separate the plasma after centrifugation (see Figure 8-21) and is ideal for samples destined for plasma chemistry panels that may sit overnight because the separation of plasma from red blood cells (RBCs) will prevent hemolysis and artifacts. The other lithium heparin tube has no separator gel and can be used for both CBCs and chemistry panels. In addition, there are plain tubes with no anticoagulant, which are used for collection of serumfor chemistry panels or serologic testing. The plain tubes also are available with a gel to separate the serum. To obtain optimum results, veterinarians should consult with technicians at the diagnostic laboratory to ensure that the samples are properly collected and processed. Poor quality samples will yield nonreliable results.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree