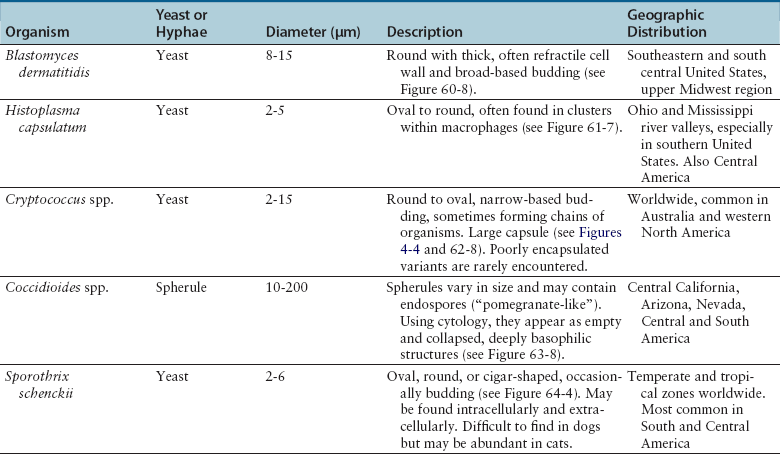
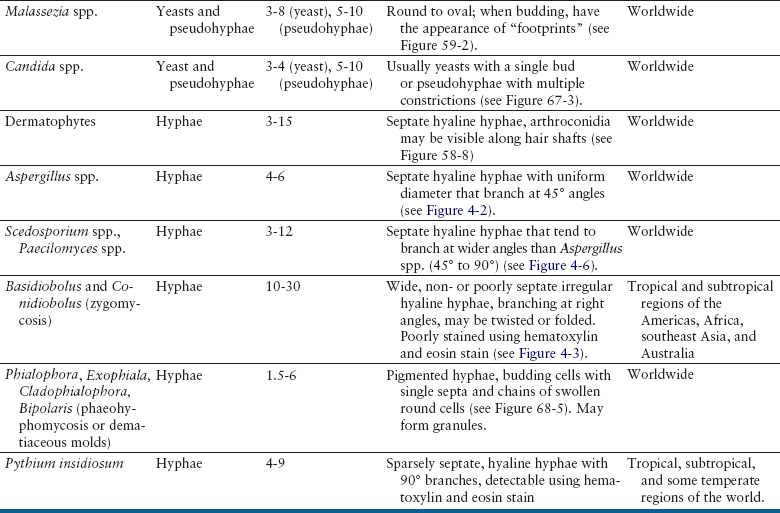
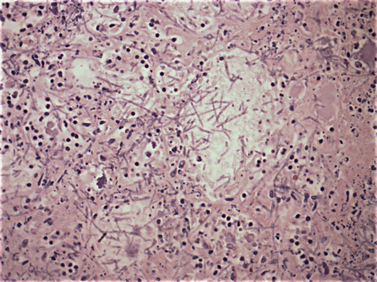
Chapter 4 • Fungi often grow more slowly than bacteria and can have specific culture media requirements. Some fungi are dangerous to culture because they produce airborne spores that may lead to laboratory-acquired infections. • The clinician should tell microbiology laboratory personnel if a fungal infection is suspected. Providing a list of suspected pathogens facilitates safe and successful culture of pathogenic fungi. The animal’s clinical abnormalities, travel history, history of immunosuppressive drug therapy, and the results of cytology or histopathology should be taken into account. • Specimens should be collected and transported as for bacterial culture. Refrigeration inhibits growth of some fungi. • Because of increasing drug resistance among pathogenic fungi, the demand for antifungal susceptibility testing has increased, and for some fungal pathogens, culture and susceptibility testing before beginning antifungal drug therapy may be advantageous. 1. Molds—molds exist as filaments known as hyphae, which may be divided into cellular compartments (septate hyphae). A mass of filaments is known as a mycelium. A granule formed by a compacted mat of fungal filaments is a eumycetoma (as opposed to an actinomycotic mycetoma; see Chapter 42). 2. Yeasts—these are single-celled organisms that reproduce by budding. In some cases, buds do not detach from the parent cell, but instead form a chain. Elongated chains of buds may resemble hyphae. These are termed pseudohyphae (Figure 4-1). FIGURE 4-1 Histopathology of the thyroid gland from a 13-year old female spayed poodle mix with disseminated Candida albicans infection. The dog had been treated with prednisone, cyclosporin, and azathioprine for refractory immune-mediated hematologic disease. Note intralesional pseudohyphae. H&E stain, 400× magnification. Fungi reproduce by producing spores. Spores can be produced following asexual or sexual reproduction. Asexual reproduction (through mitosis) generates spores that are identical to the parent cell. Sexual reproduction involves the fusion of haploid nuclei from two hyphal structures, which is followed by meiosis. The asexual stage of a fungus is known as an anamorph, whereas the sexual stage is known as a teleomorph. Many fungi have two different species names to reflect these stages. For example, the teleomorph of Cryptococcus neoformans is known as Filobasidiella neoformans. Formally, the teleomorph name is supposed to take precedence and can be used to describe both stages.1 However, asexual reproduction predominates in most fungi, including during growth in culture. Thus, certain fungi, such as C. neoformans, are more widely known by their anamorph name. In addition, teleomorphs of some fungal organisms have not yet been described. Some organisms can exist in more than one asexual form, and therefore have more than two names. For example, Pseudallescheria boydii (teleomorph) has two asexual forms, Graphium fruticola and Scedosporium apiospermum. Currently, there is a move by mycologists to identify only a single name for a fungal organism (‘one fungus = one name’). Throughout this book, the name most commonly used in the literature is used to refer to different fungal pathogens. Isolation of fungal organisms from clinical specimens can be facilitated by the use of special media. For many fungal species, this may be hazardous to laboratory personnel. Submission of specimens for culture of dangerous organisms such as Blastomyces dermatitidis, Coccidioides immitis, and Histoplasma capsulatum is discouraged if the diagnosis can be obtained using other methods such as cytology, histopathology, and serologic testing. These organisms exist as yeasts in tissues (at 37°C) that are minimally hazardous to clinicians and laboratory personnel, but grow as hyphae at lower temperatures and on culture medium, which generate spores that can be inhaled and cause disease. Specimens that are commonly submitted for fungal culture include lower respiratory tract wash specimens, cerebrospinal fluid, urine, other body fluids, skin, eye, hair, or biopsy specimens from the nasal cavity, bone, or skin. Guidelines for specimen collection are the same as for bacterial culture (refer to Chapter 3). The clinician should inform the laboratory what fungal species are suspected, because different fungi have different media preferences and growth conditions. As large a specimen as possible should be collected. Care should be taken to avoid collection of contaminated specimens. Blood cultures may be useful for dogs and cats with disseminated fungal disease when lesions cannot be easily accessed. Detection of fungemia can sometimes be achieved using blood culture systems for bacterial isolation. Lysis-centrifugation methods (Isolator tubes, Wampole Laboratories) may be more sensitive for detection of dimorphic and filamentous fungi in blood. Mycobacterial blood culture media (such as MB/BacT, BioMérieux) may also offer increased sensitivity for detection of fungemia.2 Specimens should be labeled with the patient’s name, patient number, and source of specimen, as well as the date and time of collection, and labeled, packaged, and transported according to regional and global regulations for shipping of infectious substances (see Chapter 1).3 Careful packaging and transport is particularly important for specimens suspected to contain fungal pathogens. If dangerous organisms might be present, the laboratory should be warned in advance and the specimens marked appropriately. Microscopic Examination of Direct Smears Examination of a direct smear before culture allows rapid partial or complete identification of many fungi by the laboratory (Table 4-1, Figure 4-2). This can help the clinician make initial treatment decisions and guides media selection by the laboratory. Using Gram stain, yeasts generally stain gram positive and molds tend to be gram negative.4 A potassium hydroxide (KOH) preparation may be performed to examine a specimen for dermatophyte hyphae and arthroconidia. The KOH clears keratinaceous and cellular debris, but fungal elements are left intact. If tissue granules are present, the laboratory will crush them, examine them for hyphae, and the granules can also be cultured. Fungal morphology may be altered in specimens from dogs and cats receiving antifungal drug treatment (Figure 4-4).
Isolation and Identification of Fungi
Introduction and Definitions
Specimen Collection and Transport
Diagnostic Methods
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Isolation and Identification of Fungi
Only gold members can continue reading. Log In or Register to continue