Inhalation Anesthesia in South American Camelids
Equipment
Conventional small animal anesthesia machines with single sodalime canisters usually do not contain sufficient absorptive capacity for efficient carbon dioxide removal in camelids weighing more than 70 kilograms (kg). Small animal anesthesia machines that have expanded sodalime canisters or dual canisters have sufficient absorptive capacity similar to anesthesia machines designed for use in humans. Anesthesia machines designed for use in adult horses are not recommended for use in camelids.
Intubation
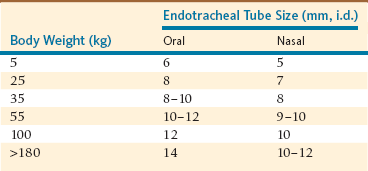
After induction, tracheal intubation is recommended because it provides a secure airway and prevents aspiration of salivary secretions and gastric fluids if regurgitation occurs. Oral intubation (cuffed tubes, 5–14 millimeters [mm], internal diameter [i.d.]) is performed as in domestic ruminants (Table 47-1). ET tube length and cuff integrity must be confirmed before intubation is attempted. Oral blind intubation is usually unsuccessful, and laryngoscopy, with a 205-mm blade for juvenile and small adult alpacas and a 350-mm blade for adult llamas, is recommended. Because the mouths of herbivores do not open very widely, the blade of the laryngoscope must be placed on the epiglottis to obtain adequate visibility of the larynx for successful intubation. Visibility of the larynx is further improved with the use of a Butler equine mouth gag to open the mouth widely, by hyperextension of the head and neck to make the orotracheal axis approach or exceed 180 degrees, and by use of a gauze sponge on a sponge forceps to swab the pharynx if secretions hinder visibility of the larynx. It is recommended that an obturator, for example, a male canine urinary catheter, be inserted through the ET tube to facilitate intubation.
Nasotracheal (NT) intubation in camelids is also possible, although it requires an ET tube one size smaller (Table 47-1). Camelids are prone to epistaxis, so use of lubricating compounds that contain phenylephrine is recommended. Blind nasal intubation is technically easier than oral intubation under laryngoscopic control. Nasal intubation under laryngoscopic control is technically more difficult than orotracheal intubation. Even though NT intubation can be more difficult, it offers the option of recovering the animal with the ET tube in place as a method of preventing airway obstruction during recovery. The ET tube is advanced through the external nares into the ventral meatus with slow gentle pressure, using the same technique used in a horse. If an obstruction is encountered at approximately 6 to 10 cm in adult llamas, it is usually because of placement of the tube in the middle meatus. If an obstruction is encountered more caudally, approximately 25 cm in adult llamas, the tube is likely in the pharyngeal diverticulum. In either case, the tube should be withdrawn and redirected. If the ET tube cannot be redirected past the pharyngeal diverticulum, placement of a prebent stylet (e.g., a piece of the smallest aluminum rod available for Thomas splints) into the tube to direct the ET tube tip ventrally is usually effective. The pharyngeal diverticulum is not as prominent in alpacas.
ET intubation may be confirmed with any of several techniques. Initially, these techniques include visualization of the ET tube passing into the larynx and absence of stertorous breathing sounds. When transparent ET tubes are used, condensation of water vapor will appear and disappear during each breath. Gas can be felt as it is expelled from the tube during exhalation. If a suction bulb (Figure 47-1) is evacuated and connected to the ET tube, it will reexpand if the tube is in the trachea and will remain collapsed if the tube is in the esophagus, thus providing immediate and definitive confirmation of correct or incorrect placement. When the ET tube is connected to an anesthesia machine, observation of synchrony between movement of the rebreathing bag and the thorax will be noted. Finally, if a capnograph or respiratory gas analyzer is available the presence of carbon dioxide (CO2) will be apparent in exhaled gas.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree