Chapter 12 Infectious diseases
Viral diseases
Foot-and-mouth disease (FMD)
Clinical features
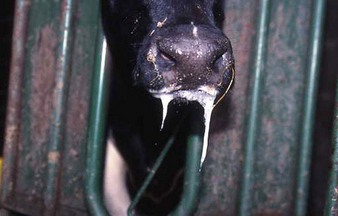
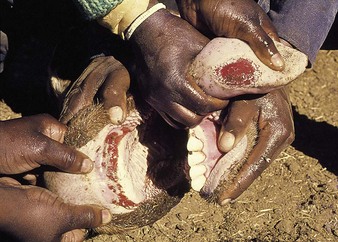
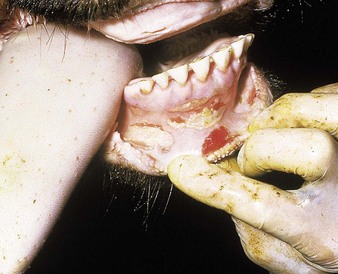
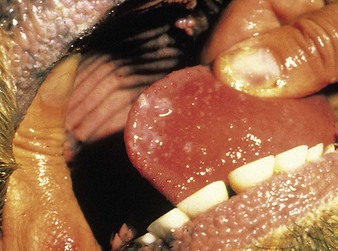
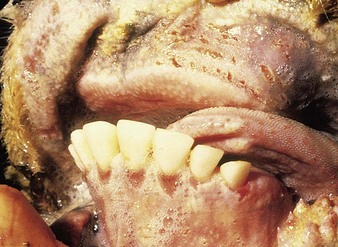
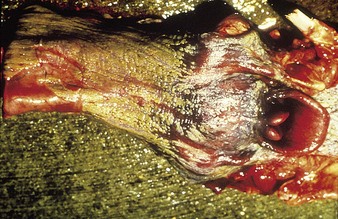
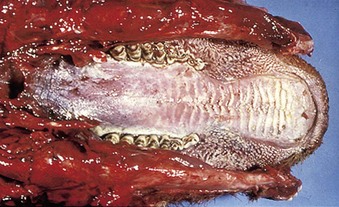
cattle infected with foot-and-mouth disease are dull, off feed, and drool saliva (12.1). Some are lame. On opening the mouth (12.2), large areas of epithelial loss, that are the result of recently ruptured FMD vesicles, are seen on the tongue and hard palate, as in this animal from Zimbabwe. 12.3 also shows recently ruptured tongue vesicles. An unruptured vesicle on the dorsum of the tongue is seen in 12.4.
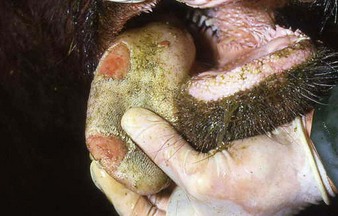
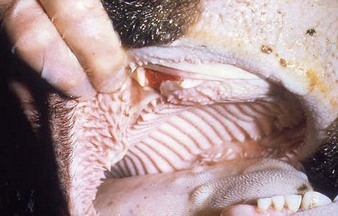
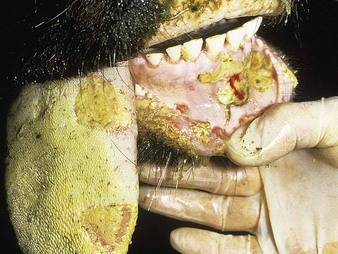
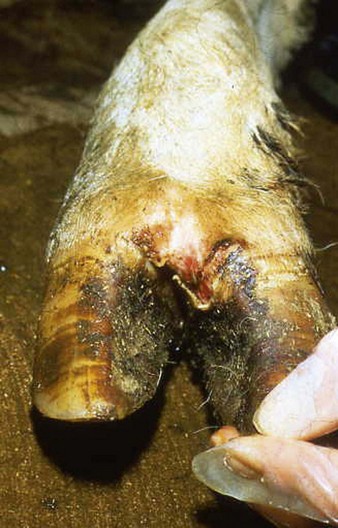
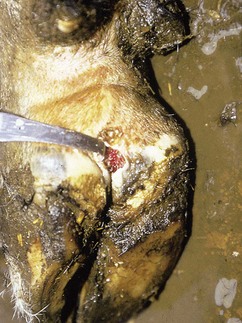
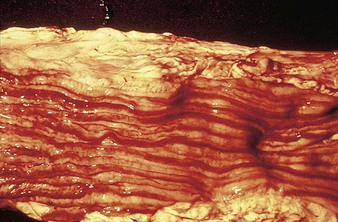
In a steer infected experimentally, within 2 days initial vesicles have ruptured to reveal ulcers which are seen along the lower gums and inside the lower lip, together with ruptured tongue vesicles (12.5). Two days later the lesions on the tongue, lower lip, and gums have become secondarily infected (12.6). Elsewhere a vesicle on the coronary band and dorsal part of the interdigital space has ruptured. On the seventh day (12.7) the interdigital space shows widespread ulceration along its entire length. A vesicle on the soft skin above the heel has ruptured in another cow (12.8). Lameness may be the first sign of FMD. These interdigital lesions easily become secondarily infected. Multiple teat vesicles are shown in 11.28 (p. 211).
Differential diagnosis
includes vesicular stomatitis (4.11), BVD/MD (4.3), bovine papular stomatitis (4.13, 4.14), rinderpest (12.13), digital dermatitis (7.57–7.60), interdigital dermatitis (7.65).
Rinderpest (“cattle plague”)
Clinical features
virgin-soil epizootics (12.9), here in Nigeria, fulminate rapidly and frequently escalate into panzootics, whereas enzootic rinderpest spreads slowly, even inapparently, for months in immature and young adult stock free of maternally-derived antibodies.
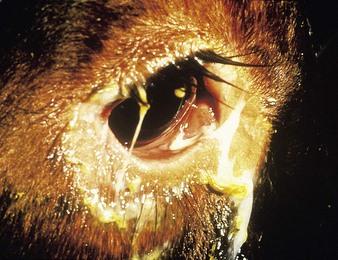
Clinical diagnosis in fresh epizootics is relatively easy: after a prodromal onset of fever, illness is evident in 48 hours. Affected animals are restless and have dry muzzles and staring coats. Milk yields fall, respirations are shallow and rapid, visible mucous membranes are congested, whilst tears (12.10) and nasal fluids (12.11) flow profusely. The appetite is impaired and constipation develops.
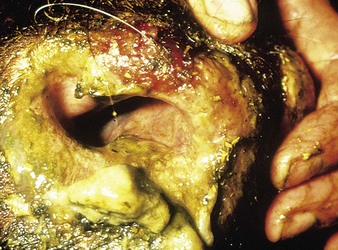
The emergence of mucosal erosions 2–5 days later is the first sign suggestive of rinderpest: raised pinheads of necrotic epithelium reminiscent of oatmeal coat all visible mucous membranes, for example tongue and lips (12.12). They are readily abraded to reveal shallow erosions with a hemorrhagic layer of intact basal cells (12.13). The erosions enlarge and coalesce throughout the alimentary tract from the mouth and esophagus (12.14) to the rectum. Salivation is profuse. The affected cattle are now obviously sick, drink excessively, and pass soft feces. In 2–3 days the fever regresses and diarrhea starts. The brown, fluid feces contain necrotic debris streaked with blood. Dehydration is rapid and the frequent straining reveals the capillary stasis in the rectum known as zebra stripes (12.15). Breathing is labored and painful. Most cattle die within 6–12 days of the onset of overt illness. Pregnant cows abort during convalescence that lasts for months. In contrast, enzootic rinderpest is difficult to diagnose clinically. Adult cows are immune and passively immunize their sucking progeny, protecting them for up to 9 months; thereafter they are at risk. The clinical signs are muted, and often one or more of the cardinal features of the classic epizootic syndrome such as fever, erosions, mucopurulent nasal and ocular discharges, and diarrhea are absent. Most affected animals survive and suspicions of rinderpest are not roused. The illustrations are from Saudi Arabia, Yemen, and Nigeria.
Differential diagnosis
lesions are indistinguishable from those in BVD (4.3). Other similar diseases are FMD (12.1–12.8), IBR (5.2), and malignant catarrhal fever (12.20–12.22).
Bluetongue (BTV)
Clinical features
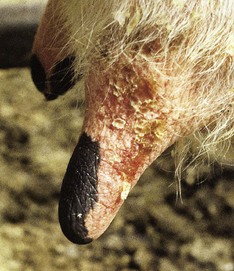
while endemic on the African continent, where it is characterized mainly by inapparent infections, bluetongue is sporadic in many other parts of the world, including Eastern Europe and the Mediterranean basin. Spread of BTV to Western European countries (including the UK, the Netherlands, and Germany) occurred in 2007. In North America it is a mild clinical condition and differential diagnosis is difficult. Infection is more common than disease, which presents as a hyperemic, inappetant animal. Bluetongue causes initial hyperemia of the muzzle and lips, followed by inflammatory and erosive lesions. This Dutch Holstein animal (12.16) shows a mucopurulent nasal discharge, hyperemia of the muzzle, and early gum erosions. Necrotic areas may be seen in the gums in a 2-year-old heifer (12.17) where the mucosa behind the incisor teeth has either sloughed or shows white diphtheritic plaques. Note the excessive salivation, a result of discomfort and a reluctance to close the jaws. Changes in the hard palate (12.18) include extensive areas of ulceration which extend onto the dental pad. Some clinical cases of BTV show irregular superficial teat erosions (12.19). Stiffness and laminitis, with distal edema of all limbs, may sometimes be seen. Clinical lesions in cattle are allegedly mediated by an IgE hypersensitivity reaction. Diagnosis is by PCR and AGID assay and by virus isolation.
Differential diagnosis
photosensitization (3.4, 3.5), BVD (4.3), IBR (5.3), vesicular stomatitis (4.11), FMD (12.2).
Malignant catarrhal fever (MCF, bovine malignant catarrh, malignant head catarrh)
Clinical features
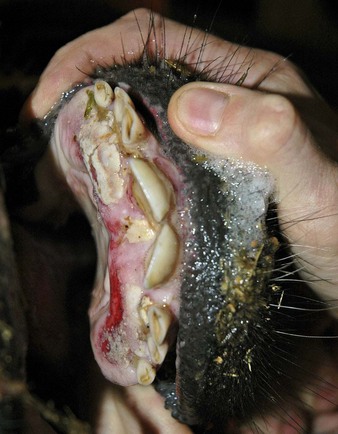
malignant catarrhal fever causes marked pyrexia, anorexia, and profound depression, with catarrhal and mucopurulent inflammation of the upper respiratory and alimentary epithelia, keratoconjunctivitis following a characteristic initial peripheral keratitis, and lymphadenopathy. Typically, a clinical case has a foul, pungent oral odor. MCF herd outbreaks are seasonal and occur predominantly in Africa. Elsewhere (North America and Europe), only sporadic cases are seen. The “head and eye” syndrome of the Devon cow in 12.20 includes a purulent oculonasal discharge, mild keratitis, and hyperemia of the nostrils. Note the almost pathognomonic hypopyon in the crossbred Charolais suckler cow in 12.21. There is a marked ocular discharge and pus is settling toward the base of the anterior chamber. Iridocyclitis may lead to photophobia. The nasal discharge is, unusually, not particularly severe. Areas of dry necrosis and ulceration are seen on the gums and dental pad of 12.22. Similar changes are commonly seen on the nostrils. Clinical cases in cattle are not infectious, but if they survive they are infected for life and may infect their calves in utero.
Differential diagnosis
rinderpest (12.10, 12.11), bluetongue (12.16–12.19), East Coast fever (12.48), IBR (5.2), BVD/MD (4.1), Jembrana disease (Indonesia) (12.57–12.60), bovine iritis (8.36).
Lumpy-skin disease (LSD)
Clinical features
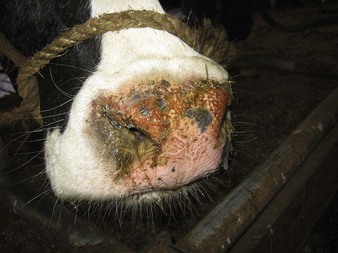
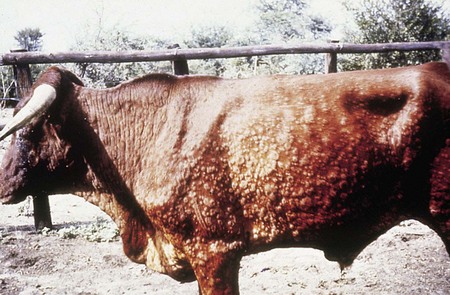
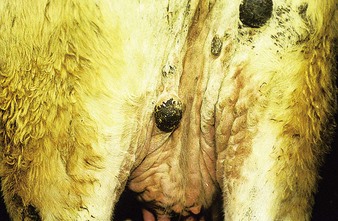
initially a fluctuating fever, lacrimation, and inappetance for 2 weeks, during which circumscribed nodules appear in the skin over the whole body (12.23) and mucous membranes of the mouth and respiratory tract, genitalia (orchitis), and conjunctiva. These nodules (12.24) are discrete, firm, raised, and painful, and contain hard gray-yellow material. Regional nodes are enlarged (prescapular in 12.24). Edema of the lower limb is common in severely affected animals. Some nodules resolve rapidly, whilst others become firm necrotic sitfasts (12.23 body and 12.25 head and neck) that heal slowly and drop out, leaving a scar. Secondary infection can lead to suppurating ulcers and abscesses. A few nodules may persist for years. Subclinical cases develop isolated nodules which are often missed.
Differential diagnosis
ulcerative lymphangitis (pseudotuberculosis) (3.48), pseudo-lumpy-skin disease (Allerton herpes virus infection) (12.26, 12.27), dermatophilosis (streptothricosis) (3.42).
Pseudo-lumpy-skin disease (LSD), (Allerton virus infection)
Definition
one of two bovine herpesvirus-2 syndromes (see also bovine herpes mammillitis, p. 208), pseudo (false)-lumpy-skin disease is characterized by transient moderate fever and exudative cutaneous plaques. Disease occurs primarily in southern Africa, and very occasionally in the USA, Australia, and the UK.
Clinical features
initial fever and mild lymphadenopathy are followed within a few days by emergence of numerous circular or oval superficial cutaneous plaques about 1–2 cm diameter. These are hard, firm, have a red margin, and enlarge to 3–5 cm, becoming umbilicated. The centers are depressed and exude to form brown crusts (12.26). The skin beneath the crusts dies within 2 weeks to reveal smooth new skin, and fresh hair grows within 2 months. Lesions are frequently on the face, neck, back, and perineum (12.27), as in this pedigree Charolais heifer in the UK. Clinical recovery is uncomplicated.
Differential diagnosis
lumpy-skin disease (12.24), urticaria (3.1, 3.2), dermatophilosis (3.37–3.43).
Rift Valley fever (RVF)
Clinical features
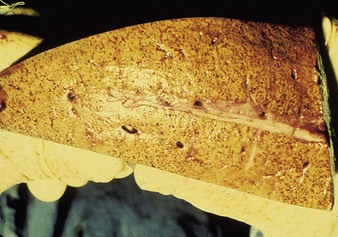
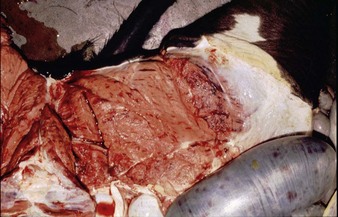
Autopsy changes in calves and fetuses include a spectacular bright orange-yellow and diffusely necrotic liver (12.28), whilst adult cattle show discrete focal necrotic hepatic lesions (12.29). Widespread hemorrhages are evident in the subcutaneous tissues, musculature, and serosa of the intestine (12.30). Diagnosis depends on viral isolation from aborted fetuses or blood. Veterinarians are at particular risk when handling infected tissues.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree