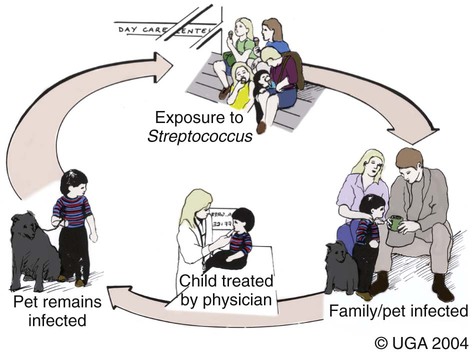
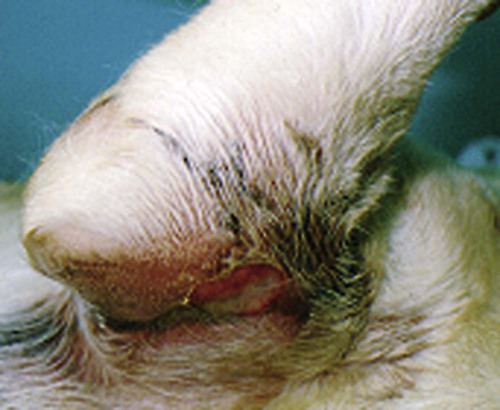
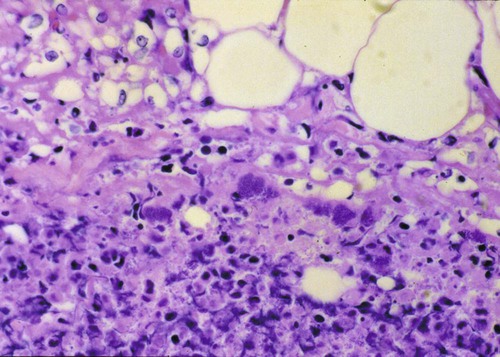
Craig E. Greene and John F. Prescott Streptococci are gram-positive, nonmotile, facultatively anaerobic cocci that cause localized to widespread pyogenic infections in animals and people. Although numerous species are pathogenic, many are commensal microflora of the oral cavity, nasopharynx, skin, and genital and gastrointestinal (GI) tracts. Species differences among streptococci are responsible for the varying host ranges and levels of virulence. Several classification systems exist for streptococci based on cultural characteristics, antigenic composition, and biochemical features. Because it tends to correlate with pathogenicity, the Lancefield classification114 based on antigenic differences in cell wall carbohydrates is used to distinguish the groups. The action on erythrocytes in culture medium has also been used to distinguish different groups of streptococci. β-Hemolysis is characterized by complete lysis of erythrocytes and clearing around the bacterial colonies. α-Hemolysis is characterized by a greenish-colored zone and intact erythrocytes in the discolored region. Some are nonhemolytic. β-Hemolytic strains tend to be most pathogenic. Lancefield groups A, B, C, E, G, L, and M are usually β-hemolytic. Group D is usually α-hemolytic but can be nonhemolytic and contains numerous species that have been reclassified as Enterococcus (see the Enterococcal Infections section in this chapter). Organisms in the α-hemolytic and nonhemolytic (γ-hemolytic) categories are found on mucous membranes and skin of clinically healthy animals. If present in an infectious process, they are usually regarded as contaminants or unimportant invaders. When they do cause illness, it may take the form of valvular endocarditis or embolic abscess. The precise species classification of streptococci in numerous disease processes described next is in doubt, especially in the older literature. Increasingly, Lancefield grouping combined with detailed phenotypic characterization by commercial identification systems classifies streptococci correctly and defines more clearly the strains responsible for significant infections in dogs and cats. People are the principal natural reservoir hosts of group A streptococci, and most human infections are caused by this group, which consists of Streptococcus pyogenes as the only member. Dermatitis, pharyngitis, and scarlet and rheumatic fevers are the main syndromes caused by S. pyogenes (Table 33-1). Rarely, these organisms produce perianal cellulitis, vaginitis, and localized abscesses in humans. Certain strains produce pyrogenic exotoxins, which may cause toxic shock–like syndrome. Of all the streptococcal groups, group A organisms have the greatest virulence for human adults, whereas organisms of the other groups, such as B, C, D, F, and G, cause the most severe manifestations in human neonates. TABLE 33-1 Summary of Streptococcal and Enterococcal Infections of People, Dogs, and Cats aB, Both dog and cat; C, cat; D, dog; H, human; UTI, urinary tract infection. bNumbered footnote symbols in this column refer to the reference list. S. pyogenes is the predominant cause of group A infections of people and is the type species for this group. The sites of greatest carriage of group A streptococci in people are the caudal aspects of the pharynx and tonsillar region. Group A streptococci can survive extremes in environmental temperature and humidity; however, most infections are associated with direct or close contact among susceptible individuals (Fig. 33-1). Shedding occurs most commonly through respiratory droplets or oropharyngeal secretions. As is true with many streptococcal infections, some individuals can harbor the infection for extended periods in the absence of clinical illness. Prevalence rates for group A streptococci are higher in young children, especially those in daycare or classroom situations. The prevalence of positive throat culture findings in such circumstances may approach 50%, even in the absence of an obvious epidemic.78 Symptomatic rather than carrier children are most likely to bring infection into the home. Under such circumstances the isolation rate in other people in the household approaches 25% to 50%. If the child is asymptomatic, the rate is only 9%.96 Dogs and cats have been suggested as possible sources of reinfection of treated household members, but no convincing evidence shows that dogs and cats are significant reservoirs of infection for people. However, veterinarians may need to be consulted on this topic. Whenever streptococcal typing has been performed on the oropharyngeal regions of dogs and cats, groups G, C, L, and M have been present (in order of decreasing frequency). However, screening for group A streptococcal colonization of the tonsils of dogs and cats from random households in urban environments has shown the apparent prevalence to range between 1% and 10%.33,36,109,138 In one study in which recurrent group A streptococcal pharyngitis occurred in people, the prevalence for households was said to be 42% for dogs and 36% for cats.33 However, because only bacitracin susceptibility was used to distinguish group A from non–group A strains in many of these studies, these findings of high prevalence must be regarded as outdated. When Lancefield typing has been done, the prevalence of true group A streptococcal carriage has varied from 0% to 3% of dogs and has not correlated with the presence of infection in the owner.36,242 Biochemical testing of isolates from dogs and cats cannot accurately distinguish between those from groups A and G.169 There is no convincing evidence that dogs or cats are a source of S. pyogenes for people, although the possibility cannot be completely dismissed, but the suggestion still persists in the less well informed medical literature.176,177 When domestic pets come into close contact with infected individuals, some pets may apparently develop pharyngeal colonization with group A streptococci. Infected pets show no clinical illness or tonsillar enlargement and usually lose their infection within 2 to 3 weeks after they are removed from the household. Infected people are carriers of group A streptococci for much longer periods.171 It is not acceptable to consider culturing and treating a dog and/or cat in a household in which reinfection occurs without doing so for the human contacts. See Chapter 99 for a further discussion of this zoonotic infection in humans. The spectrum of effective antimicrobials for treatment of group A streptococcal infection in pets is the same as that for human strains. It is judicious to treat pets when they may be a source of recurrent infection of household members. Isolates of group A streptococci from dogs have shown the greatest susceptibility to penicillin, erythromycin, and chloramphenicol. Recommended total daily dosages are listed in Table 33-2. Resistant strains can be treated with cephalosporins. TABLE 33-2 Drug Therapy for Streptococcal Infections in Dogs and Catsa IM, Intramuscular; IV, intravascular; PO, by mouth; SC, subcutaneous. aFor specific dosages for perinatal group G infections in cats, see Table 33-3. bFor additional information, see the Drug Formulary in the Appendix. cDose per administration at specified interval, expressed as mg/kg unless otherwise stated. Streptococcus pneumoniae can produce pneumonia, bacteremia, otitis media, endocarditis, and meningitis in people but is of uncertain significance in dogs and cats. Although it possesses no Lancefield antigens, it is mentioned in this section because, like S. pyogenes, it is almost exclusively a human pathogen. A single report of bacteremia and septic arthritis in a cat attributed the infection to transmission of S. pneumoniae from a human infant in the same household.194 Necrotizing fasciitis caused by S. pneumoniae was reported in a cat that received trauma while playing with children.249 A French bulldog developed hyperesthesia and central vestibular disease from meningoencephalitis caused by S. pneumoniae.93 Group B streptococci (Streptococcus agalactiae) have been associated mainly with neonatal septicemia and postpartum metritis in people and mastitis in cows. Skin, pharyngeal, and wound infections can occur. Immunosuppressed individuals can develop disease in many tissues (see Table 33-1). Group B streptococci are more frequently isolated than group G from people with these syndromes. Factors at the time of delivery, such as low birth weight or difficult delivery, precipitate the development of clinical illness. Group B streptococcal infections are rare in dogs and cats. They have been reported to cause septicemia in a dog, endometritis, and “fading puppy syndrome,” symptoms of which include bacteremia, pyelonephritis, and necrotizing pneumonia. Group G streptococci have been far more commonly isolated than group B as causes of neonatal sepsis. Similarly, peritonitis with septicemia and parturient endometritis and placentitis have been described in cats. Whether canine and feline strains are indigenous or of human or other animal origin is uncertain. In one dog, the organism was isolated from the skin and may have been a transient resident from a human source.64 Treatment is similar to that for group A streptococcal infections (see Table 33-2). Disease associated with group C streptococci has been described only in dogs (although dogs and cats may carry these organisms as commensal flora) in lower frequency than group G. Identification of group C streptococci in the lower respiratory tract washings of clinically healthy dogs and dogs with chronic infectious respiratory infections in kennels was associated with more severe respiratory disease in infected populations.27 Histologic analysis revealed that dogs with group C streptococci were more likely to have intra-alveolar neutrophils than those without this infection. Chronic bilateral nasal discharge, of 7 months’ duration, was described in a 10-month-old dog where S. equi ssp. zooepidemicus was isolated.158a Histopathologic abnormalities of nasal biopsy specimens were chronic diffuse lymphoplasmacytic rhinitis. Treatment was instituted with a combination of amoxicillin-clavulanate and marbofloxacin and nebulization with 0.9% sodium chloride. Improvement was gradual during the 1 month of therapy with eventual resolution of disease. The organism could not be isolated after therapy was discontinued. The dog had been exposed to horses on a stud farm, which may have been a source of its infection. In cats, reports of upper respiratory infection have also been described. Two adult cats housed in separate animal shelters developed rhinitis and acute (in less than 24 hours) fatal meningitis associated with infection by S. equi ssp. zooepidemicus.21a Acute hemorrhagic and purulent pneumonia has been described in dogs as a cause of acute respiratory infection with group C organisms. These outbreaks have been primarily in racing greyhounds or confined colony dogs.* The isolates have been confirmed as Streptococcus equi ssp. zooepidemicus in most cases. This organism, along with Streptococcus canis, is uncommonly found as a nasal commensal in dogs. It is a common commensal of the skin and respiratory tract in horses and can be an opportunistic pathogen. Its isolation from three dogs was associated with their close contact with horses.2a In that report, one dog had pneumonia, another was a clinically healthy dog in close contact, and the third dog, from another household, had chronic rhinitis like the dog in the previous paragraph. Increased isolation prevalence of S. equi ssp. zooepidemicus in the lower respiratory tracts of dogs has been associated with tracheobronchitis and pneumonia. Mildly affected animals have coughing and gagging but continue to eat and drink. In severely affected animals, weakness, coughing, dyspnea, fever, hematemesis, and red urine have been the predominant clinical signs. Many of the dogs developed septicemia, and some died suddenly without signs of clinical illness. Gross lesions at necropsy of diseased animals consisted of widespread petechial and ecchymotic hemorrhages and pulmonary congestion with mediastinal and free pleural hemorrhage. Microscopically, streptococci were found in clusters intracellularly throughout the lung parenchyma and in the spleen. Histopathologic findings are fibrinonecrotic and hemorrhagic pneumonia. Higher levels of mRNA for proinflammatory cytokines (interleukin [IL]-8, IL-6, and tumor necrosis factor [TNF]-α) in affected dogs’ tissues as compared to control dogs’ tissues suggested an exuberant inflammatory response similar to toxic shock (see Invasive Infections, later).163a A similar outbreak of respiratory disease in a large (~700-cat) cattery was attributed to infection with S. equi ssp. zooepidemicus.18a Affected cats had purulent nasal discharges, coughing, dyspnea, signs of pneumonia, and death, similar to what has been reported for dogs. Inflammatory lesions were present throughout the respiratory tract. Severe acute diffuse bronchopneumonia was found in most of the 39 cats necropsied, and a few had pleuritis or peritonitis. Pyogranulomatous meningoencephalitis, similar to what was reported in cats with upper respiratory disease, was found in 4 cats. S. equi ssp. zooepidemicus was the predominant organism isolated from the lesions. The circumstances leading to group C streptococcal septicemia or pneumonia in adult dogs kept in kennels or in groups are unclear, and these infections appear to be rare. S. equi ssp. zooepidemicus is, however, well recognized as a virulent and broad host-range pathogen, and a report that isolates from one shelter outbreak were clonal158 suggests that a virulent strain spread among animals, which might either have not been previously exposed to the organism or may have been predisposed by intercurrent viral or bacterial infection (e.g., canine infectious respiratory disease), or both. Early combined treatment with penicillin and an aminoglycoside has been effective in resolving the pneumonia.104 Co-infection of canine influenza virus (CIV) and S. equi ssp. zooepidemicus is incriminated as increasing the severity of either pathogen alone (see Chapter 23).180a There is in vitro evidence that influenza virus infection increases the incidence and severity of bacterial complications associated with streptococcal infection.79a Vaccination of dogs against CIV infection reduced the severity of clinical illness and influenza virus replication in both CIV-challenged, and CIV plus S. equi ssp. zooepidemicus–challenged, dogs (see Chapter 100).180a,180b There is one report of a S. equi ssp. zooepidemicus infection in a person acquired from an infected dog.1b The person was a dog handler, in close contact with the infected animal, and genetic analysis confirmed an identical organism in the dog and person. S. equi ssp. equi, the etiologic agent of strangles, has been isolated from a dog with tonsillar and submandibular lymphadenomegaly caused by pharyngeal foreign body penetration.110 Genetic characterization confirmed the isolate as this usually highly equine-specific pathogen. The dog lived on premises where horses were kept; unexpectedly, no clinical cases of strangles had been identified in the horses in the local environment. Other group C streptococci isolated from less severe infections (urinary tract infections [UTIs] or abscesses) in dogs have been identified as Streptococcus dysgalactiae ssp. equisimilis, although this subspecies also has caused serious losses through septicemia and pneumonia in captive coyotes.71 S. dysgalactiae ssp. dysgalactiae has been described as an unusual cause of neonatal septicemia in puppies.225 Drugs and dosages recommended for treatment are similar to those for group A infections (see Table 33-2). Group D streptococci are considered normal GI flora of dogs, and many of these species have been classified as enterococci. They have been isolated from the tonsils and other tissues of cats, dogs, and other domesticated animals. These enteric cocci are often found in UTIs or nosocomial tissue infections of dogs. Streptococcus suis has been isolated rarely from pleuropneumonic and dermatologic conditions in cats48 but appears to be of little significance. However, S. suis was isolated at necropsy from a cat with pyogranulomatous meningoencephalitis.173 It has also been isolated from the urine of dog with fever and bacteruria.148a The clinical signs resolved within 48 hours of instituting treatment with cephalexin. This dog had no known exposure to swine; however, it had been allowed to chew on pig ear treats. β-Hemolytic streptococci are commensal microflora of, and are associated with lesions of, the skin, pharynx, and upper respiratory and genital tracts of cats (Web Table 33-1). The majority of β-hemolytic streptococcal infections in cats are caused by Lancefield group G streptococci and are apparently S. canis. Whether more virulent disease-producing strains of this organism exist is uncertain, but these organisms can cause severe infections in kittens. Neonatal kittens are infected with the streptococci from the vagina of the queen. Streptococci can gain entrance via the umbilical vein and can spread by direct extension into the peritoneal cavity or through the ductus venosus and portal circulation of the liver, resulting in bacteremia. In juvenile kittens (3 to 7 months old), cervical lymphadenitis may follow a subclinical episode of pharyngitis and tonsillitis. Group G infections of older cats are often opportunistic and result from wounds, trauma, surgical procedures, viral infections, and immunosuppressive conditions. These suppurative infections can result in septicemia and embolic lesions, most often in the lung and heart. WEB TABLE 33-1 Anatomic Distribution of β-Hemolytic Streptococcal Isolatesa from Lesions in Cats aOf 38 isolates tested, 34 were Lancefield group G positive. bNumber of times isolated in pure culture (only organism isolated). Data compiled from cases during a 17-year period at the Veterinary Medical Teaching Hospital, University of California, Davis, Davis, CA. The clinical signs vary with the site of infection and host immunocompetence. Sites of streptococcal infections in cats are listed in Table 33-1 and Web Table 33-1. Although cats of any age may be affected, most cases involve neonatal kittens (younger than 2 weeks of age). Most infected kittens gain less weight than littermates, and occasionally an affected kitten has a swollen, infected umbilicus. Death usually occurs by 7 to 11 days of age, but kittens born to queens with minimal prior exposure may die suddenly at younger than 3 days of age with overwhelming sepsis. In kittens with septicemia, the febrile response is transient, occurs within 24 hours before death, and frequently remains undetected. Older kittens are febrile and anorexic with swelling and purulent exudate at the site of infection. Cervical lymphadenitis is a unilateral or bilateral swelling in the ventral cervical lymph nodes (Fig. 33-2). Abscess, pharyngitis, pneumonia, diskospondylitis, osteomyelitis, and arthritis are other localizations of the infectious process in this age group.89 Animals with respiratory localization with pneumonia have fever, anorexia, coughing, and dyspnea. Concurrent dermal ulceration of the face or extremities, necrotizing fasciitis, rhinitis, necrotizing sinusitis with subsequent frontal and ethmoid bone erosion can occur. Progression to toxic shock–like syndrome, sepsis, and death has also been reported.157 The same report describes suppurative meningitis after skin ulceration and chronic respiratory infection in outbreaks, among cats of all ages, that were densely housed in shelters. As noted earlier, infected cats can also develop bacteremia and subsequent diskospondylitis characterized by fever, paraspinal hyperesthesia, and progressive paresis. Fever, joint swelling, and lameness are characteristic of arthritis and may involve one or more joints or extremities. Gram staining of exudates from affected tissues reveals single and chains of gram-positive cocci. Confirmation is based on bacteriologic culture of the affected tissues. Aerobic culture of exudates or needle aspirates of enlarged lymph nodes yield S. canis. In fatally affected neonates the organism is found most consistently in the liver, lung, umbilicus, and peritoneal cavity (Fig. 33-3). Necropsy findings in affected neonatal kittens with septicemia include omphalophlebitis, peritonitis, and less frequently, embolic hepatitis, pneumonia, diskospondylitis, and myocarditis. Untreated cases of cervical lymphadenitis in juvenile cats can progress to embolic myocarditis and embolic pneumonia with secondary pulmonary infarction and pleuritis and pyothorax.246 Valvular endocarditis has also been described.132 Group G streptococci are very sensitive to penicillin and its derivatives. Juvenile and older cats with lymphadenitis should be treated immediately with oral or parenteral therapy (Table 33-3). Draining and flushing the abscesses hasten recovery. These cats should be examined for predisposing conditions such as feline leukemia, feline infectious peritonitis, feline immunodeficiency virus or viral respiratory infections, feline urologic syndrome, and wounds. TABLE 33-3 Therapy for Streptococcus canis Infections in Cattery Cats Based on Age A, Adults; IU, international units; J, juveniles; N, neonates; Q, queens. aFor additional information, see the Drug Formulary in the Appendix. bDose per administration at specified interval. cTotal dose needed for each drug in a fixed combination, based on 2- to 3-kg cat. This drug has been associated with injection-site sarcomas. dOnly one or two doses are usually given during this treatment regimen. Treatment of infected juvenile or adult cats with lymphadenitis or arthritis can be accomplished with parenteral or oral therapy (see Table 33-3). Doses are higher than normally recommended because the organism can be harbored in the tonsillar crypts, and a considerable amount of pus forms in the abscess. Cats with diskospondylitis have required up to 6 months of therapy. Parenteral therapy can be instituted in the veterinarian’s office, and medication can be dispensed for subsequent oral administration. Group G streptococci have received increasing attention as a cause of pharyngitis, tonsillitis, wound infection, cellulitis, neonatal and puerperal septicemia, and endocarditis in people. Group G streptococci in cats appear to be S. canis and seem to be distinct from human group G streptococci.169 In a review of all reported human group G infections in Great Britain, a majority of the patients had underlying immunosuppressive illness.89 S. canis, apparently originating from a cat and poor sanitary practices, was responsible for an unusual outbreak of mastitis in dairy cows.219 Raw milk from such dairies would be a source of human infection. Group G streptococci are the major streptococcal type isolated as commensal flora from the skin and mucosa of dogs.17,18 The majority of group G isolates from dogs are S. canis. Nevertheless, some group G isolates have the biochemical characteristics of human group G streptococci. More work is needed to define the biotypic variation within S. canis and the identity of unusual group G streptococci isolated from dogs. Historically, the veterinary literature has contained numerous reports of diseases in dogs caused by these organisms, including abortion, infertility, sterility, and neonatal death. In addition, cellulitis, keratitis, mastitis, pharyngitis, arthritis, pericarditis, tonsillitis, pneumonia, polysynovitis, necrotizing fasciitis, endocarditis, cholangiohepatitis, meningoencephalitis, and genital infections have been described (see Table 33-1). Metaphyseal osteomyelitis may occur in growing pups.66 Some of the historical descriptions of disease, especially pharyngitis, tonsillitis, infertility, and sterility, caused by group G streptococci should be carefully considered because of the frequency with which these organisms are isolated from clinically healthy animals and the lack of clear description of the microbiology of the infections. However, it is also likely that, as in other animals and in people, β-hemolytic streptococcal infections have declined in importance in the past 50 years with the widespread use of penicillin and its derivatives. S. canis is generally an opportunistic pathogen of dogs and is isolated from an array of nonspecific infections, including genitourinary tract, wound, mammary gland, and skin (especially otitis externa). In addition, it may cause bacteremia or septicemia and polyarthritis in neonatal puppies, forming part of the fading puppy syndrome, in which the pup is predisposed because of lack of warmth, improper navel disinfection, and lack of nursing. Infection in these pups may come from microflora or the environment; no correlation was found between the organisms found in the milk of bitches with those isolated from dead septicemic pups.179 Group G streptococci have also been associated with a rapidly progressive systemic infection in older dogs (see streptococcal toxic shock syndrome discussion in this chapter). S. canis is a common commensal of the genital tract. It may be an opportunistic organism associated with vaginitis, but its historical role in infertility in bitches is not supported by current findings. Historical but no current accounts exist of the association of this organism with acute tonsillitis and suppurative cervical lymphadenitis in dogs in kennels and other settings. Group G streptococci have also been isolated from the cerebrospinal fluid (CSF) of dogs with meningoencephalomyelitis as a result of hematogenous spread or penetrating injuries.28 Polymerase chain reaction (PCR) has been used to detect these organisms in CSF of a dog with bacterial meningoencephalitis.142 Space-occupying abscess formation may occur within the confines of the central nervous system (CNS) in some cases. Results of CSF analysis are marked pleocytosis (usually greater than 100 cells/µL) with a predominance of neutrophils and in some cases, intracellular bacterial cocci. Antimicrobial choices for group G infections are similar to that for group A streptococcal infections (see Tables 33-2). Organisms of this group are generally susceptible to erythromycin, clindamycin, and penicillin (as well as other β-lactams). Aminoglycoside susceptibility is variable. Although highly effective, vancomycin use is not recommended, because it is reserved for resistant human infections. For CNS infections, therapeutic options include high-dose intravenous penicillins, trimethoprim-sulfonamide, clindamycin, or intravenous third-generation cephalosporins (see Bacterial Infections of the Central Nervous System, Chapter 91). The past decade has had a resurgence of severe invasive group A human streptococcal infections characterized by septic shock associated with multiple organ failure and with or without necrotizing fasciitis and myositis (NFM) (Box 33-1).87 In dogs and cats, S. canis is usually responsible for this syndrome. With NFM, bacteria at the inoculation site synthesize proteolytic and other products that facilitate the organism’s spread via fascial planes and into muscles. Periosteal involvement, adjacent to involved muscle, has also been reported.108 S. canis isolates that resisted phagocytosis were examined for virulence genes comparable to those found in group A (S. pyogenes) human isolates. Only M-protein genes associated with surface fibrillar material and streptolysin O genes were identified in most of the canine isolates.50 Increased invasiveness of streptococci correlates with a change in the M-protein, which inhibits phagocytosis by neutrophils and macrophages. Once the streptococci develop this new protein, they can enter the body and avoid host defenses. Multiple organ involvement in streptococcal toxic shock syndrome (STSS) suggests that a toxin produced by the pathogenic bacteria might also be involved in the pathogenesis.164 Bacterial superantigens, a family of highly mitogenic proteins secreted by the organisms, are suspected. Superantigens simultaneously bind to major histocompatibility complex class II molecules and T-cell receptors, resulting in sudden high cytokine levels. In vitro, enrofloxacin (a quinolone) caused bacteriophage-induced lysis of an S. canis strain that had been isolated from a dog with STSS.92 Bacteriophage induction was associated with an enhancement of expression of a superantigen gene relative to the same organism in noninduced cultures. The presence of this gene on enrofloxacin-induced bacteriophages may explain the association between this drug treatment and the occurrence of STSS in dogs. Most of the dogs reported with this type of infection had received enrofloxacin in the early stages of disease; however, the drug was not effective in halting the progression of the disease, despite in vitro susceptibility in some cases. In fact, enrofloxacin may have been responsible for triggering the pathologic events caused by these virulent streptococci. The dramatic and severe multisystemic STSS and locally invasive NFM group A streptococcal infections in people have been dubbed toxic shock infections and flesh-eating bacterial infections, respectively, in the popular media. A similar clinical picture caused by various isolates of S. canis has been identified in dogs* and cats.157,202,206,234,249 In most cases, affected animals were previously healthy adult dogs or cats younger than 1 year of age. The dogs had a history of mild trauma, bite wound, or respiratory disease or UTI. Affected kittens had suppurative lymphadenomegaly typical of group G infection. In addition, they had multifocal purulent ulcerative skin lesions. They were generally depressed but afebrile.234 All the dogs were febrile (40° C to 41° C [104° F to 105.8° F]) at admission. Dogs with NFM had severe, rapidly developing cellulitis, usually of a limb, but in one case it occurred in the ventral thorax. The most consistent clinical sign in the history and on physical examination was intense, excruciating pain localized around the affected area but sometimes involving the whole body. Localized heat and swelling were identified at presentation or within 2 days of hospitalization. Once evident, the cellulitis developed rapidly (Fig. 33-4). A left shift, with variable leukocyte count and toxic granulation, is frequent. Serum creatinine kinase activities are usually increased in cats with muscle involvement. Colonies of streptococci in chains can be readily demonstrated in fluid aspirated from the cellulitis or in underlying tissues or internal organs by histopathologic examination (Fig. 33-5). Pulmonary interstitial or alveolar densities resulting from bacterial or hypercoagulable emboli may be detected with thoracic radiography. Cytologic examination of fine-needle aspirates from swollen areas is essential to speed confirmation of infection and institute early appropriate antimicrobial therapy, because virulent strains of gram-negative bacteria can also cause this syndrome.244 Bacteriologic culture should also be performed on portions of the aspirated material; however, results are often delayed. Dogs with NFM were extremely depressed when examined and eventually went into shock. They had extensive exudate accumulation along fascial planes. The fascia required surgical debridement. In two dogs, acute onset of posterior paresis with lower motor neuron deficits developed because of the presence of septic emboli in blood vessels of the gray matter of the spinal cord and because of extension of infection into the surrounding connective tissue, muscle, and peripheral nerves. Other dogs with severe invasive S. canis infection developed STSS without clinically apparent NFM. The dogs with STSS had severe depression, fever, and rapidly developing hypotension and shock. In most, the lungs were considered the primary site of infection, and acute infection appeared to be superimposed on chronic preexisting pulmonary infection. In one dog a UTI was the presumed source.144 Similarly, cats with STSS that did not develop NFM had bacterial embolic localization with lesions in the lungs, heart, and liver.206 Some cats with NFM responded favorably to treatment with amoxicillin-clavulanate, supportive care, and wound management with topical antibacterial application, whereas in other cases, the cats were moribund or dead.234 The dogs with STSS died or were euthanized within 48 hours of hospitalization, but those with NFM survived. All dogs had full-thickness skin sloughs and required aggressive debridement of necrotic tissue, incision of fascia of affected muscles, appropriate antibacterial treatment, and intensive supportive medical care (crystalloids, colloids, plasma, low-dose heparin) aimed at treating shock with disseminated intravascular coagulation. Intravenous plasma has been used in people to control hypotension and neutralize bacterial toxins.103 Intravenous IgG therapy has also shown some benefit in clinical improvement and reduction of mortality in treated versus control human patients.39,198 Less commonly, glucocorticoid treatment was used. More appropriate antibacterials for treatment of this condition are penicillin G, aminopenicillins (ampicillin, amoxicillin), erythromycin, and clindamycin. In human STSS or NFM, clindamycin is regarded as the drug of choice. In addition to its antibacterial activity, clindamycin is a potent suppressor of bacterial toxin synthesis, inhibits M-protein synthesis (which facilitates phagocytosis), and suppresses lipopolysaccharide-induced monocyte synthesis of TNF.196 The authors (CEG and JFP) have found this drug to be valuable in the treatment of affected pets. Surviving animals often must have extensive skin grafting or dermoplasty to close open wounds created by the necrotic process. In general, public health risks from S. canis colonization of infection in dogs or cats are low. Only rarely have people been infected with this organism.69,233 Nevertheless, when veterinarians drain and debride NFM lesions, they should recognize the extreme risk of inadvertent infection of cuts on their hands. This risk can probably be contained by wearing latex gloves and protective clothing when handling dogs with STSS or NFM. One report has been made of this syndrome in a person; the source was group G streptococci.227 Group G streptococci are normal inhabitants of the skin, oropharynx, GI tract, and female genital tract of people but are rarely S. canis. Asymptomatic pharyngeal carriage of group G streptococci is found in up to 23% of people. The organisms commonly colonize human skin, and approximately 5% of asymptomatic puerperal women harbor them on the genital mucosa. Group G streptococcal infections in people are not common and involve primarily dermatitis and pharyngitis; however, bacteremia and septicemia, endocarditis, peritonitis, peripheral sepsis, soft-tissue infection, and septic arthritis are unusual. There is no evidence that dogs and cats are a significant source of infection for humans,21,69 although human infection with S. canis presumably acquired from animals may sometimes occur In this report, dogs were mentioned as a possible source of group G streptococcal arthritis in people, but the association was speculative. The source of infection was more likely autogenous. In one report of meningitis,95 the isolate was not completely characterized. In another report, S. canis was isolated from the blood of a person with septicemia.13 The organism was specifically typed on the basis of biochemical and genetic analysis. It was presumably transmitted from the family dog and colonized the varicose ulcers of the person’s legs. Similar wound infections were reported in three dog owners.112 One had concurrent S. canis bacteremia and another had concurrent infection with Pasteurella multocida. The occurrence of such infections probably involves close contact between the animal and open skin wounds. Routine hand washing after animal contact and not allowing dogs to lick people, especially their wounds, are always advisable.
Gram-Positive Bacterial Infections
Streptococcal Infections
Group A Streptococcal Infections of Dogs and Cats
Species (Serogroup)
Host Speciesa
Microfloral Distributionb
Disease Syndromesb
STREPTOCOCCUS
S. pyogenes (A)
H
Tonsils
Tonsillitis, pharyngitis, otitis, impetigo, bacteremia, toxemia,15 toxic shock, necrotizing fasciitis32
H
None (human reservoir)
Asymptomatic
S. pneumoniae (A)
H
Tonsils
Pneumonia, otitis, bacteremia, polyarthritis, meningitis
C
None (human reservoir)
Polyarthritis, bacteremia,194 necrotizing fasciitis249
S. agalactiae (B)
H
Anorectum, vagina
Neonatal: sepsis53
Immunosuppressed: bacteremia, meningitis, endocarditis67,124
Postparturient: metritis; septic arthritis; pharyngitis; respiratory, skin, and wound infection124
D
Urogenital region
Fatal septicemia in pups, necrotizing pneumonia, bacteremia, endocarditis, pyelonephritis64,106,106
C
Urogenital region
Peritonitis, septicemia, placenitis54
S. equi (C)
D
Upper respiratory tract of horses
Tonsillar and submandibular lymphadenomegaly110
S. equi spp. equi (C)
H
Upper respiratory tract of horses
Meningitis159a
S. equi ssp. zooepidemicus (C)
D
Skin, urogenital region (horse reservoir)
Endocarditis, septicemia, upper respiratory tract disease,2a,158a acute fibrinopurulent bronchopneumonia, death,24,27,70,72,104,158,163a UTIs
C
Skin, urogenital (horse reservoir)
Upper respiratory tract disease, meningoencephalitis21a
H
Skin, urogenital region (horse reservoir)
Meningitis, bacteremia, infection of many organs58a
S. dysgalactiae ssp. equisimilis (C)
H
None (animal reservoir)
Pharyngitis, glomerulonephritis,167 pericarditis
D
Mucosae
Pneumonia and septicemia in coyotes71
S. dysgalactiae ssp. dysgalactiae (C)
D
Urogenital region
Fatal septicemia and embolic bacterial pneumonia in pups225
S. suis (D or none)
C
Oropharynx
Dermatitis, fibrinonecrotic pleuropneumonia,48 meningoencephalitis173
D
Mucosae
Urinary tract infection148a
S. milleri group (S. intermedius [F])
H, D
Skin, mucosae
Opportunistic infections, abscesses in many tissues58
S. canis (G)
C
Nasopharynx, genitalia, skin
Abscesses, neonatal sepsis, umbilical infections,11,89,76,246, pyelonephritis, rhinitis, sinusitis, meningitis, necrotizing fasciitis, toxic shock, endocarditis112,132,145,157
D
Tonsils, anorectum,130 genitalia
Otitis media, neonatal sepsis (fading puppy?), umbilical infections, polyarthritis, abscesses, dermatitis, meningoencephalitis,28166b cholangiohepatitis,152a mastitis,40,44,44 genital infections: infertility, anestrus, abortion, failure to conceive,224a endocarditis,205a UTI,181 pericarditis,192 streptococcal toxic shock syndrome/necrotizing fasciitis,50,51,108,160 keratitis220a
H
None (dog, cat reservoirs)
Cutaneous ulcers,112 arthritis,21 septicemia13,69
Streptococcus sp. (L)
D
Genitalia
Abortion, fading puppy syndrome, sterility in bitch, endometritis147
Streptococcus sp. (M)
D
Tonsils
Asymptomatic colonization220b
Streptococcus sp. (E)
D
Skin, upper respiratory tract136
Asymptomatic colonization found as mixed flora in mucosal inflammation
ENTEROCOCCUSc
E. faecalis, E. avium, E. faecium (all D)
D, H, C
Intestine, feces,136 tonsils
Asymptomatic colonization, urinary tract infection, nosocomial surgical infections, endocarditis85,217
E. hirae (D)
D
Intestine, feces
Diarrhea, symptomatic upper intestinal colonization47,150a
C
Intestine, feces
Enteritis,cholangitis, pancreatitis116

Streptococcus pyogenes
Drugb
Species
Dosec (mg/kg)
Route
Interval (hours)
Duration (days)
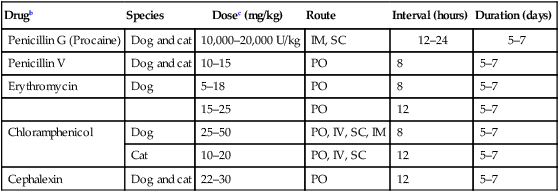
Penicillin G (Procaine)
Dog and cat
10,000–20,000 U/kg
IM, SC
12–24
5–7
Penicillin V
Dog and cat
10–15
PO
8
5–7
Erythromycin
Dog
5–18
PO
8
5–7
15–25
PO
12
5–7
Chloramphenicol
Dog
25–50
PO, IV, SC, IM
8
5–7
Cat
10–20
PO, IV, SC
12
5–7
Cephalexin
Dog and cat
22–30
PO
12
5–7
Streptococcus pneumoniae
Group B Streptococcal Infections of Dogs and Cats
Group C Streptococcal Infections of Dogs and Cats
Group D Streptococcal Infections
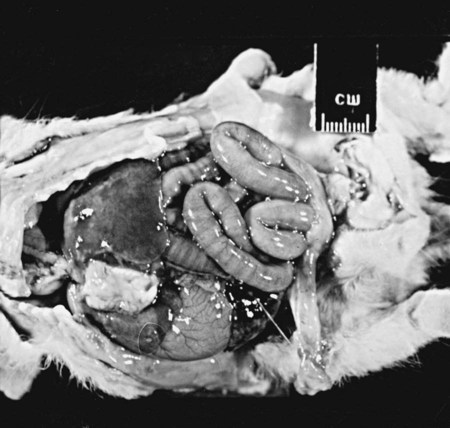
Group G Streptococcal Infections
Cats
Source
Number of Isolates
Number of Pure Isolatesb
Integumentary system
57
23
Respiratory system
38
11
Genital (female) tract
28
12
Urinary tract
17
4
Serous cavities
22
9
Neonatal sepsis
12
6
Otherc
34
13
TOTAL
208
78
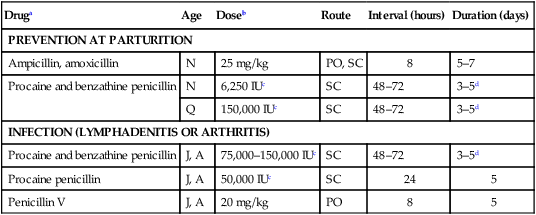
Druga
Age
Doseb
Route
Interval (hours)
Duration (days)
PREVENTION AT PARTURITION
Ampicillin, amoxicillin
N
25 mg/kg
PO, SC
8
5–7
Procaine and benzathine penicillin
N
6,250 IUc
SC
48–72
3–5d
Q
150,000 IUc
SC
48–72
3–5d
INFECTION (LYMPHADENITIS OR ARTHRITIS)
Procaine and benzathine penicillin
J, A
75,000–150,000 IUc
SC
48–72
3–5d
Procaine penicillin
J, A
50,000 IUc
SC
24
5
Penicillin V
J, A
20 mg/kg
PO
8
5
Dogs
Invasive Infections: Streptococcal Toxic Shock Syndrome and Necrotizing Fasciitis and Myositis
Public Health Considerations
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Gram-Positive Bacterial Infections